Research
Development of Catalytic Asymmetric C–H Transformation Reactions
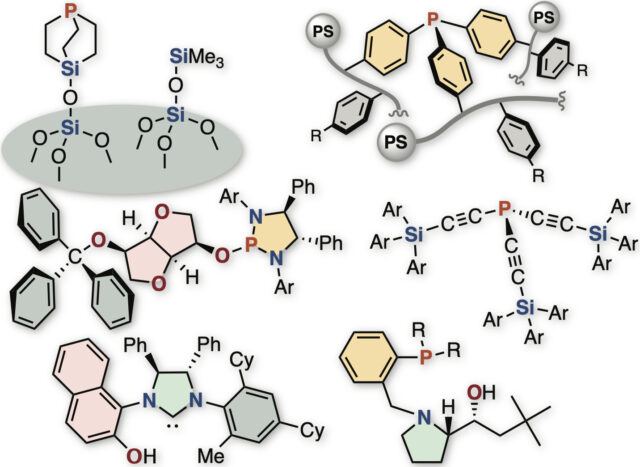
Catalytic and site-selective C–H bond transformations of organic molecules is a powerful method for accessing complex compounds through short-step synthetic processes. In direct transformation of methylene C–H bonds having different substituents (R1–CH2–R2), depending on which of two carbon-hydrogen bonds is converted, the product becomes a chiral compound with a mirror image molecular structure such as right and left hands. With a focus on weak interactions that act between catalysts and chemical substances, we are working on the development of new asymmetric synthesis methods to obtain pure chiral compounds through direct transformation of carbon–hydrogen bonds.
Development of Photon-Driven Transformative Chemical Reactions
Promotion of a chemical reaction requires energy supply. Heat is a common energy source in common chemical reactions. In contrast, we are trying to develop entirely new chemical reactions that make use of “light” as an energy source. Since light can provide much higher energy than heat, we will be able to realize novel reactions that were ever thought to be impossible. However, the molecule activated by the high energy of light is prone to decompose via unexpected side reactions. We are developing transformative chemical reactions by controlling behaviors of the activated molecules by molecular design and by collaborating with metal catalysts.
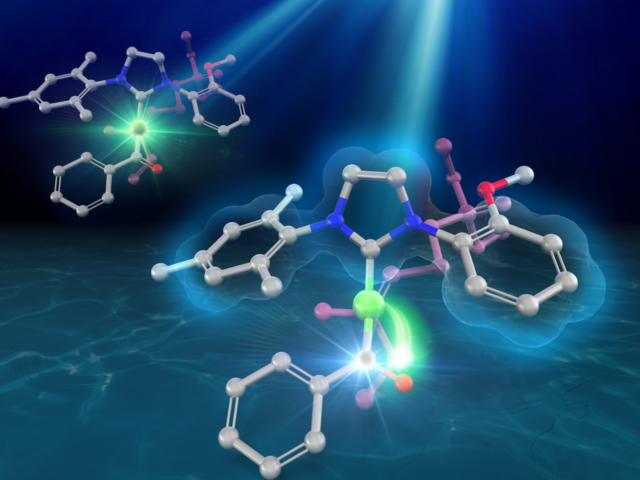
Quantum Chemical Calculations for Designing Asymmetric Synthesis Catalysts
As molecular creators, we have designed various catalysts and developed revolutionary asymmetric reactions, driven by our knowledge, experience, and imagination. In addition to this, we have been developing innovative asymmetric synthesis catalysts by elucidating the working principles of the catalysts through the use of quantum chemical calculations, and applying the knowledge gained from these calculations to the design of new catalysts. By maximizing the use of quantum chemical calculations, including the cutting-edge AFIR method developed by Professor Maeda in the Theoretical Chemistry Laboratory, we are able to take a rational approach to the creation of high-performing catalysts.