Abstracts from articles presented by members of the Lab
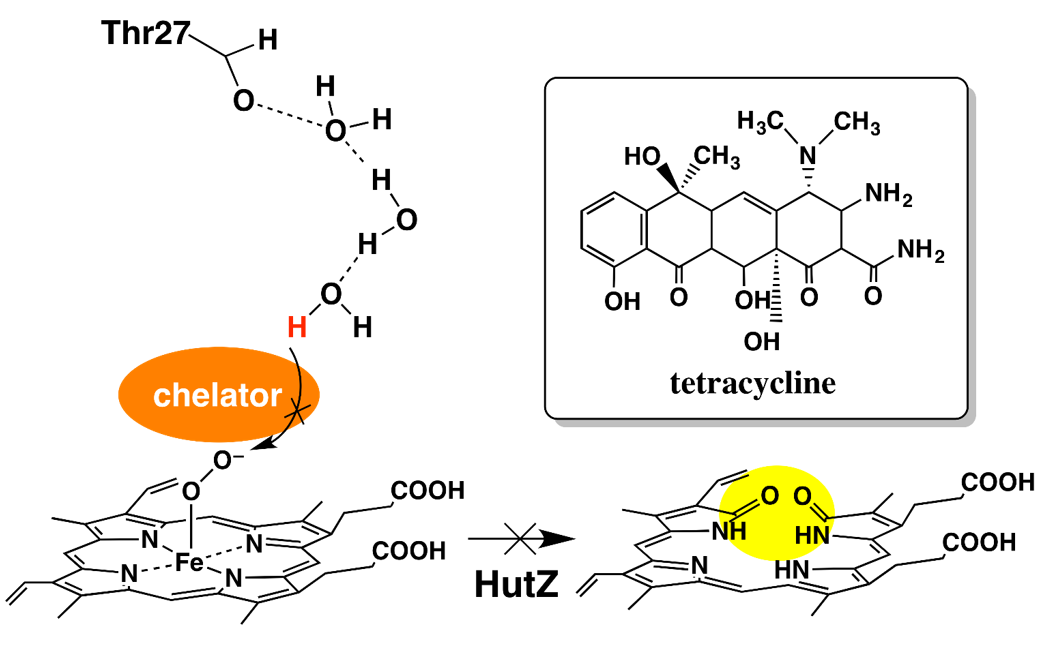
Iron chelators inhibit the heme-degradation reaction by HutZ from Vibrio cholerae, Nobuhiko Dojun, Yukari Sekine, Koichiro Ishimoria and Takeshi Uchida, Dalton Trans., 2017,46, 5147-5150
HutZ is a heme-degrading enzyme. We found that the heme-degradation reaction by HutZ is inhibited by the iron chelators. Kinetic analysis of each heme-degradation step suggests that water molecules hydrogen bonded to Thr27 are involved in proton transfer to Fe(III)–OO−, and that this step is inhibited by iron chelators.
A heme degradation enzyme, HutZ, from Vibrio cholerae, T. Uchida, Y. Sekine, T. Matsui, M. Ikeda-Saito, K. Ishimori, Chem.Commun., 48, 6741 (2012)
Cholera is one of the major infectious diseases. Cholera germs that enter the body by oral ingestion proliferate in the intestines and the cholera toxin produced by them causes diarrhea, vomiting and other symptoms. The germs require iron ion for proliferation as they use iron-containing proteins to produce energy. Since the germs that infected humans acquire iron from their hosts, elucidation of the acquisition mechanism is an important task that will lead to the prevention of infection. As approximately 70% of iron in the human body exists in the form of heme in hemoglobin, heme serves as the main source of iron for cholera germs. The germs use a group of proteins known as Hut to take in heme. Genetic analysis revealed that a type of protein known as HutZ is a heme degradation enzyme. Therefore, its heme degrading activity was examined by developing a HutZ expression system, and it was found that HutZ degrades heme via an intermediate known as verdoheme, which is characteristic to human heme degradation enzymes, even though it has no amino acid sequence identity with such enzymes. It was also found that a hydrogen-bond network that does not exist in other heme degradation enzymes existed near the active center and that enzyme activity was controlled by changing the strength of the hydrogen bond.
Structural features of small benzene clusters (C6H6)n (n≤30) as investigated with the all-atom OPLS potential, H. Takeuchi, J. Phys. Chem. A, 116, 10172 (2012)
Little progress has been made in research on the structure of the benzene cluster, which is the simplest aromatic hydrocarbon cluster. This paper presents a structure obtained by using OPLS potential. The most stable structure and many low-energy structures were found by a structural perturbation heuristic method developed by the author. Characteristics of the cluster structure and the cluster growth process were examined. Structural features were extracted at the time using local structure analysis and rotational constants. It was found that, while the basic structure in a cluster of atoms exhibiting isotropic interaction is an icosahedron, the basic structure of a benzene cluster is remarkably distorted due to the anisotropy of intermolecular interaction. Looking at the pair distribution function of benzene, the distribution of its cluster was highly anisotropic unlike the almost isotropic distribution in the liquid state. It was assumed that structures with different orientations of molecules were coexisting in this case, since the existence of multiple structures with significantly varied positions of molecules was not confirmed in the interpretation of the results of a past experiment on clusters in a supersonic jet.
NMR basis for interprotein electron transfer gating between cytochrome c and cytochrome c oxidase
Koichi Sakamoto, Masakatsu Kamiya, Mizue Imai, Kyoko Shinzawa-Itoh, Takeshi Uchida, Keiichi Kawano, Shinya Yoshikawa, and Koichiro Ishimori
The final interprotein electron transfer (ET) in the mammalian respiratory chain, from cytochrome c (Cyt c) to cytochrome c oxidase (CcO) is investigated by 1H-15N heteronuclear single quantum coherence spectral analysis. The chemical shift perturbation in isotope-labeled Cyt c induced by addition of unlabeled CcO indicates that the hydrophobic heme periphery and adjacent hydrophobic amino acid residues of Cyt c dominantly contribute to the complex formation, whereas charged residues near the hydrophobic core refine the orientation of Cyt c to provide well controlled ET. Upon oxidation of Cyt c, the specific line broadening of N-H signals disappeared and high field 1H chemical shifts of the N-terminal helix were observed, suggesting that the interactions of the N-terminal helix with CcO are reduced by steric constraint in oxidized Cyt c, while the chemical shift perturbations in the C-terminal helix indicate notable interactions of oxidized Cyt c with CcO. These results suggest that the overall affinity of oxidized Cyt c for CcO is significantly, but not very much weaker than that of reduced Cyt c. Thus, electron transfer is gated by dissociation of oxidized Cyt c from CcO, the rate of which is controlled by the affinity of oxidized Cyt c to CcO for providing an appropriate electron transfer rate for the most effective energy coupling. The conformational changes in Lys13 upon CcO binding to oxidized Cyt c, shown by 1H- and 1H, 15N-chemical shifts, are also expected to gate intraprotein ET by a polarity control of heme c environment.

