Research Understanding the regulation mechanisms of innate immune response by PPM1D phosphatase
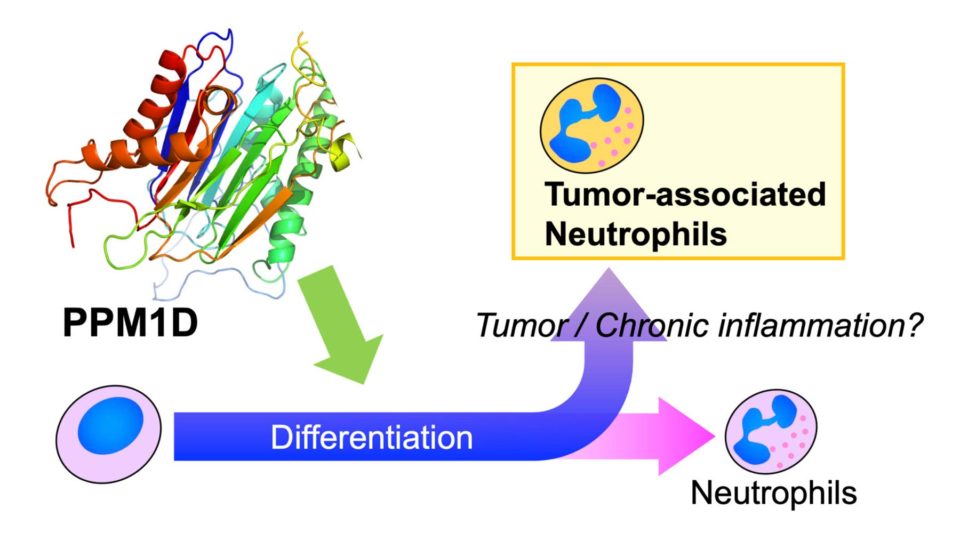
Neutrophils are the most abundant white blood cells, which function in the early stages of innate immunity to protect from the pathogens. Neutrophils have heterogeneous subsets with different functions. Among these subsets, there are immunosuppressive subsets which is accumulated in cancer tissues. We are working to clarify the molecular mechanism of polarization to these immune suppressive subsets for the establishment of more effective cancer immunotherapies.
Overexpression and gene mutations of PPM1D have been frequently observed in leukemia and myelodysplastic syndrome. PPM1D knockout mice exhibit immunodeficiency, spermatogenic disorders, and metabolic disorders. These data suggest that PPM1D plays an important role not only in carcinogenesis but also in immune responses and metabolism. In our laboratory, we focus on Ser/Thr phosphatase PPM1D and are working to understand the differentiation and immune response of immune cells and the relationship between immune cells and cancer. We have discovered PPM1D430, a splicing variant of PPM1D; this variant has been demonstrated to be expressed specifically in the leukocytes and testes. We have reported that inhibition of PPM1D induces neutrophil differentiation. We have shown that PPM1D and its splice variant PPM1D430 exhibit different localization in neutrophils, suggesting that leukocyte-specific variant PPM1D430 has distinct function for neutrophils.
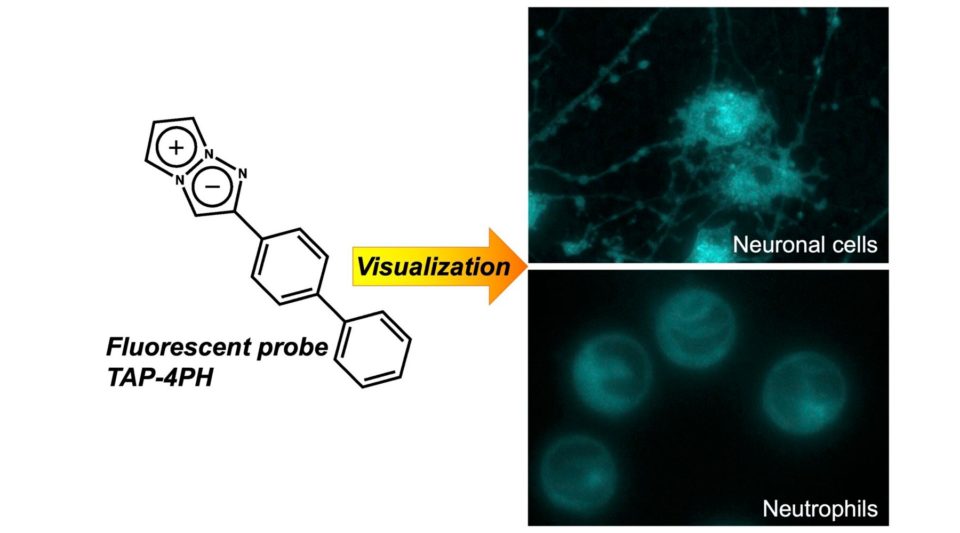
We have also developed a new method for visualizing cell differentiation and stimulus response using novel fluorescent probes, 1,3a,6a-Triazapentalene derivatives, which allow is to analyze protein functions in cell.
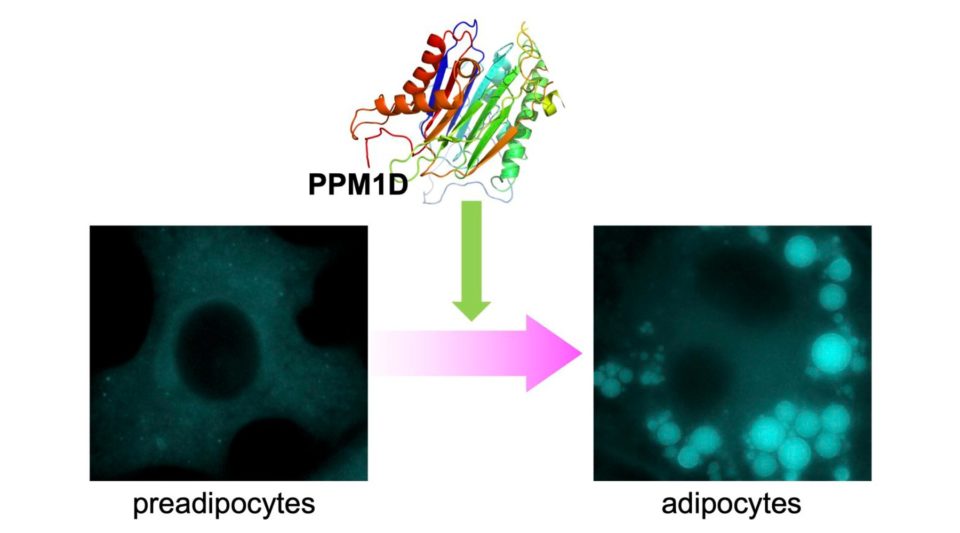
We are also working to understand the role of PPM1D on adipocyte differentiation and lipid droplet formation, which are closely related to the immune response. Obesity is a significant risk factor for various severe diseases including diabetes, hypertension, and cancer. Obesity is induced by adipocyte hypertrophy and an increase in adipose tissue mass. Thus, it is strongly required for understand the regulation mechanism of adipocyte differentiation and maturation. We have reported that PPM1D regulates white adipocyte differentiation and lipid droplet formation. Treatment of PPM1D specific inhibitor, SL-176 dramatically decreased the adipocyte differentiation, indicating that PPM1D specific inhibitor is a promising lead compound for anti-obesity drug developments. In our laboratory, we are working to elucidate the mechanism of lipid droplet formation and function of PPM1D in beige adipocyte formation.