Research Tumor suppressor protein p53: From oligomer formation to cell cycle control, and more.
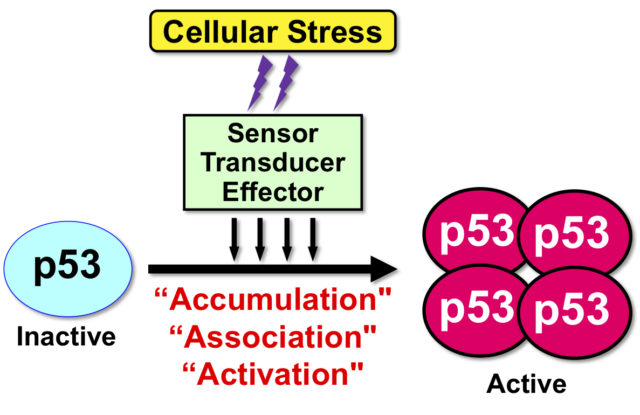
“Tumor suppressor protein p53” plays a central role in preventing tumor development. Therefore, the p53 gene TP53 is found most frequently in malignant tumors. p53 is involved in cellular response to a wide variety of cell crises, such as oxidative stress, viral infection, and starvation stress as well as genotoxic stress with DNA damage. In other words, p53 is the most important protein for maintaining the integrity of the genome in individuals and tissues (Fig. 1).
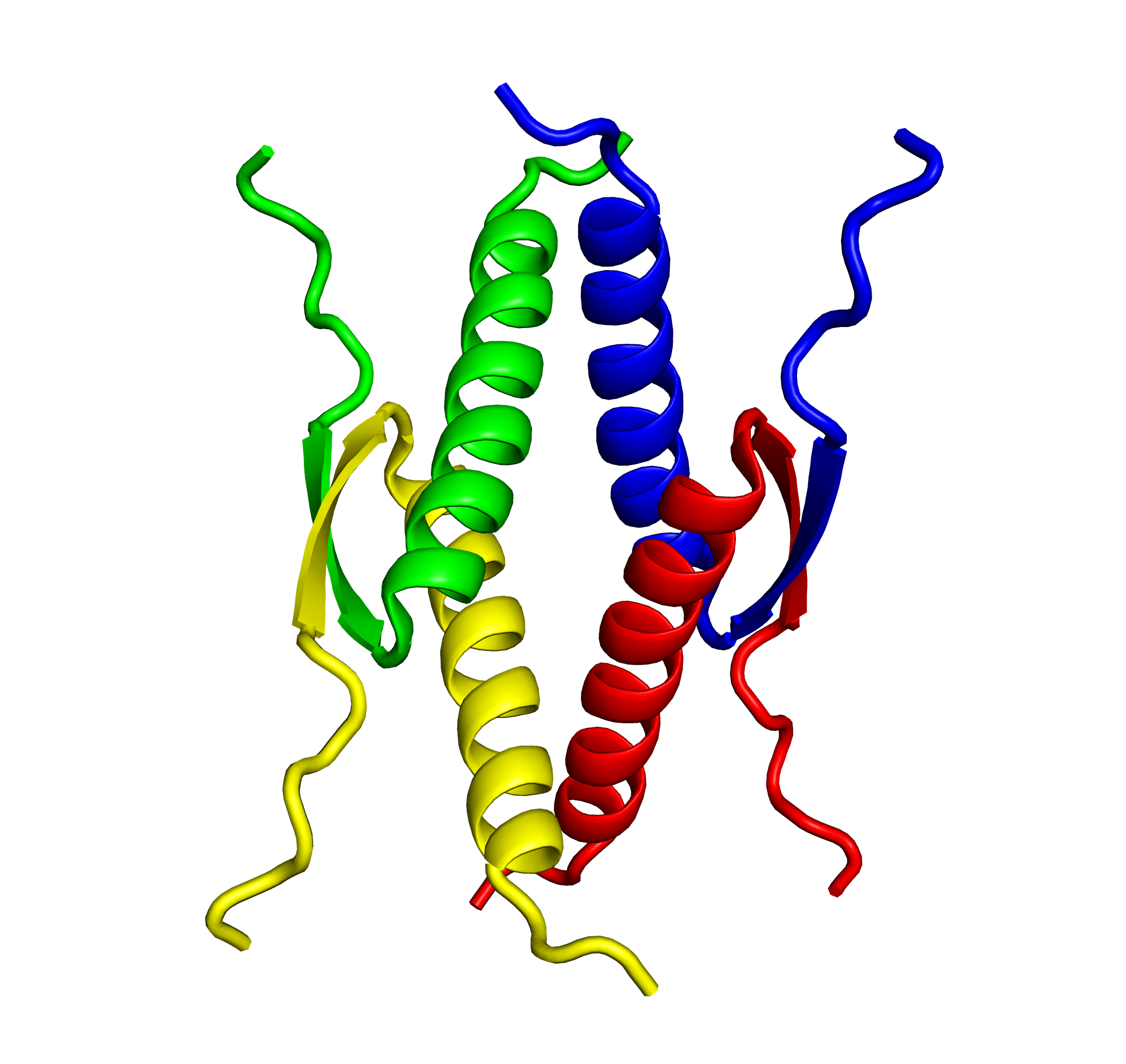
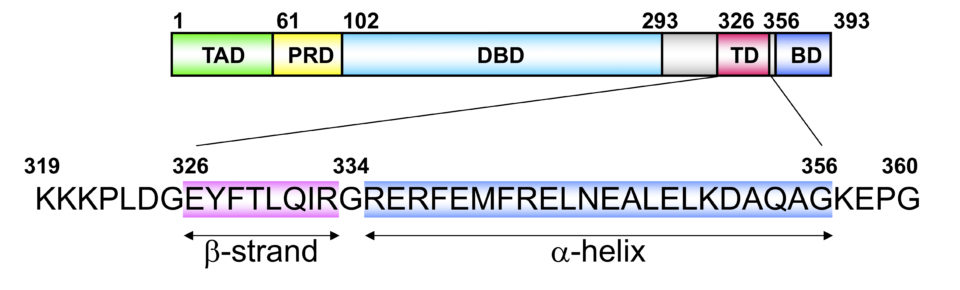
Tetramer formation is essential for the p53 functions. The tetramer formation domain of p53 is located in the C-terminal region (the residues of 326-356). and consists of a β-strand (326-333), turn (334), and α-helix (335-356) structure. Two dimers form a unique tetramer via antiparallel β-sheets and a 4-helix bundle (Fig. 2 and 3). The function of p53 is precisely regulated by the regulation of interaction with other proteins, oligomer formation, stability and localization through various post-translational modifications such as phosphorylation and acetylation.
In our laboratory, we are conducting comprehensive research study on “Tumor suppressor protein p53” from the structure to function, and also from evolution to biotechnology, particularly based on the tetramer formation, which is essential for the p53 function.
We have analyzed the oligomer formation and thermodynamic stability of the mutant tetramerization domains of p53 found in various tumors. The mutations induce significant destabilization or even abolition of tetramer formation, resulted in p53 dysfunction. We have also successfully developed a compound that is able to stabilize the mutant tetramer structure, and a hyper-stable analog by introducing an unnatural amino acid. On the other hand, it is known that p53 activity inhibits iPS cell establishment and genome editing. In order to solve these problems, we are developing a method to temporarily arrest the endogeous p53 function through the regulation of p53 tetramer formation.
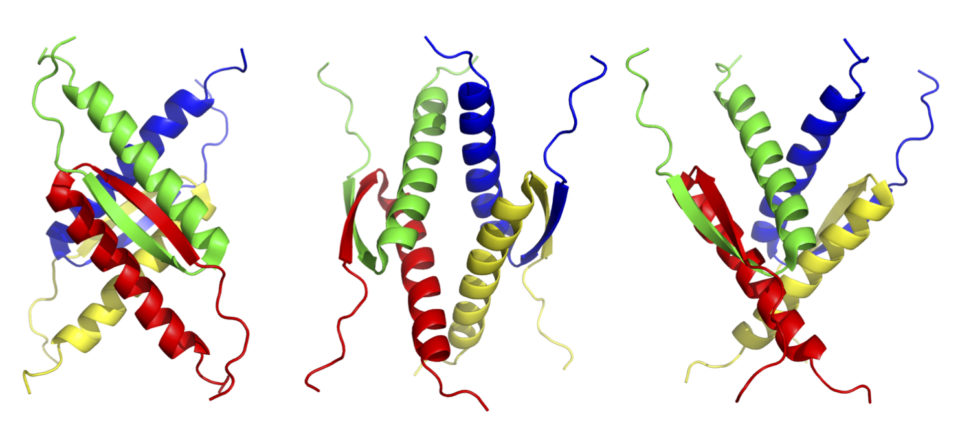
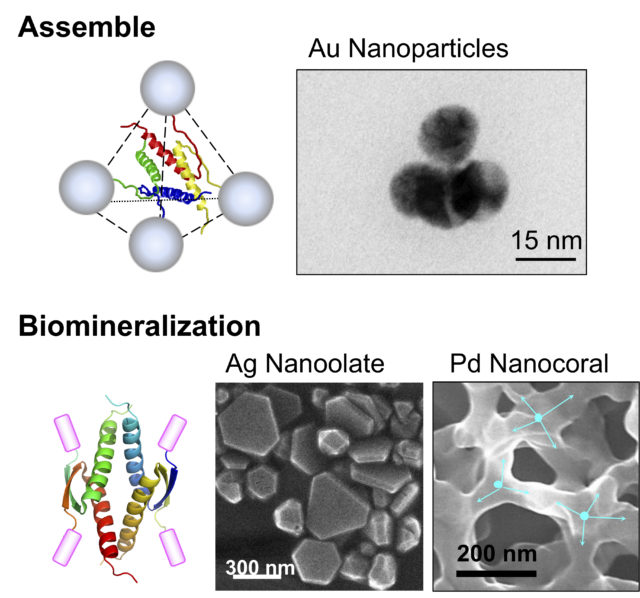
In addition, the unique structure of the p53 tetramer domain can be used as a molecular element that arranges various functional molecules in a specific three-dimensional position. We have shown that this molecular element drastically enhanced biological activities of biomolecules such as biomineralization (Fig. 4).
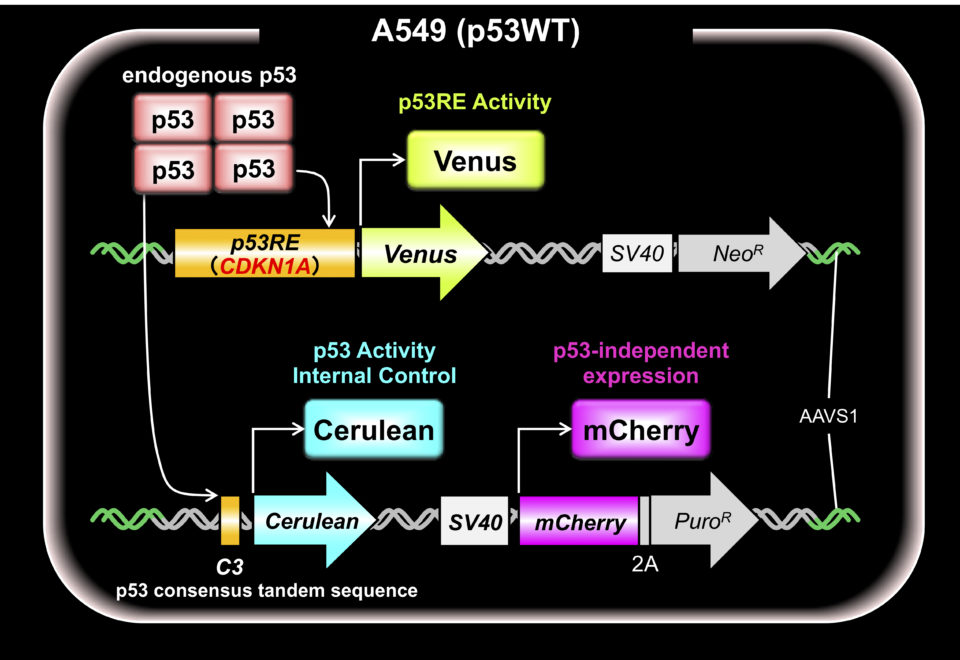
Quantitative analysis of endogenous p53 activity at the physiological concentration in living cells is very important to analyze the p53 function. Therefore, we have developed a monitoring system using multiple fluorescent proteins (Fig. 5). The system allows us to analyze p53 tetramer formation, normal and mutant hetero-tetramer formation in early-stage cancer cells, dominant negative effects and selectivity for downstream target genes.