Research Protein phosphatase PPM family: Novel functions, regulation, substrate recognition, and development of new inhibitors
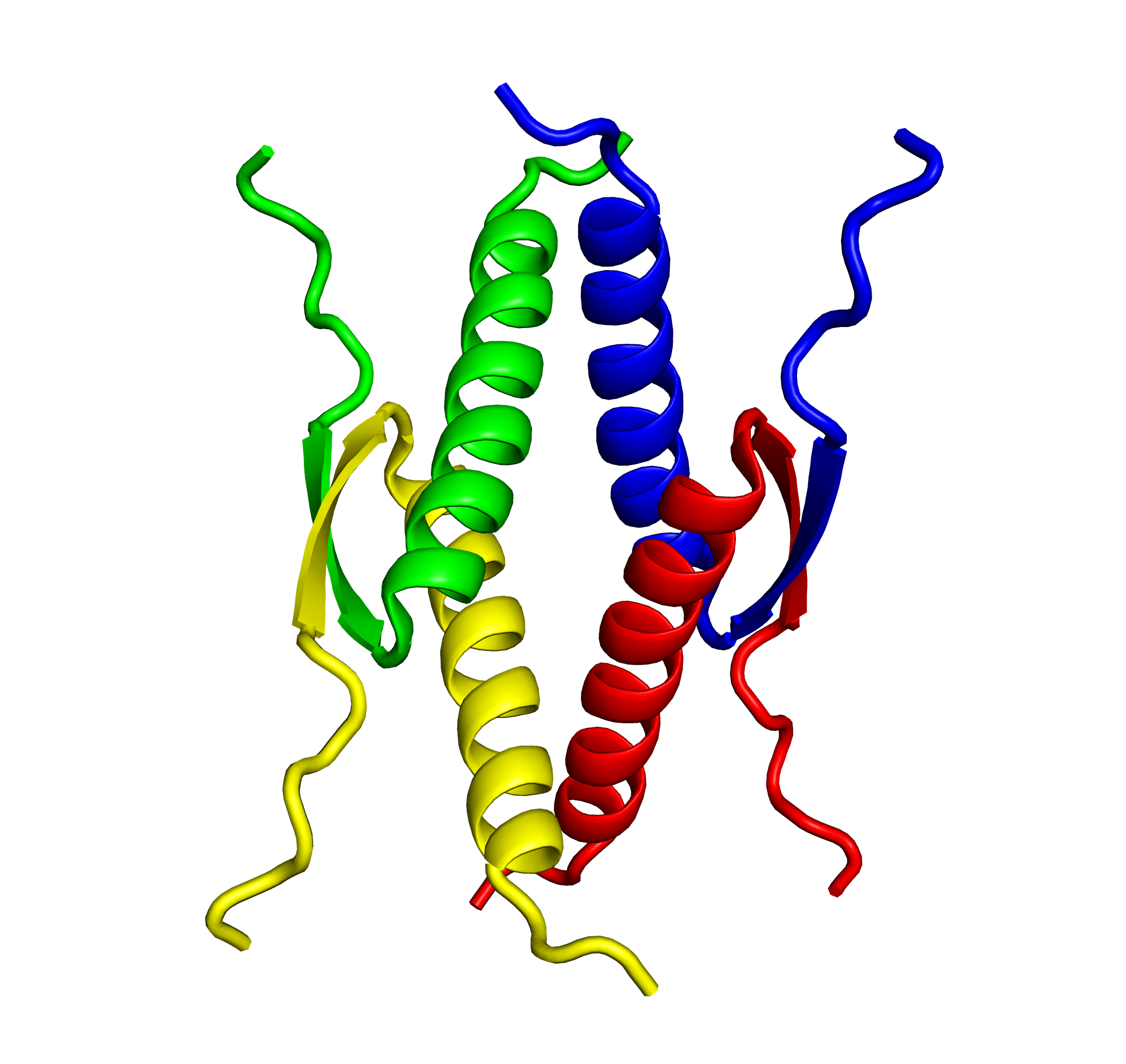
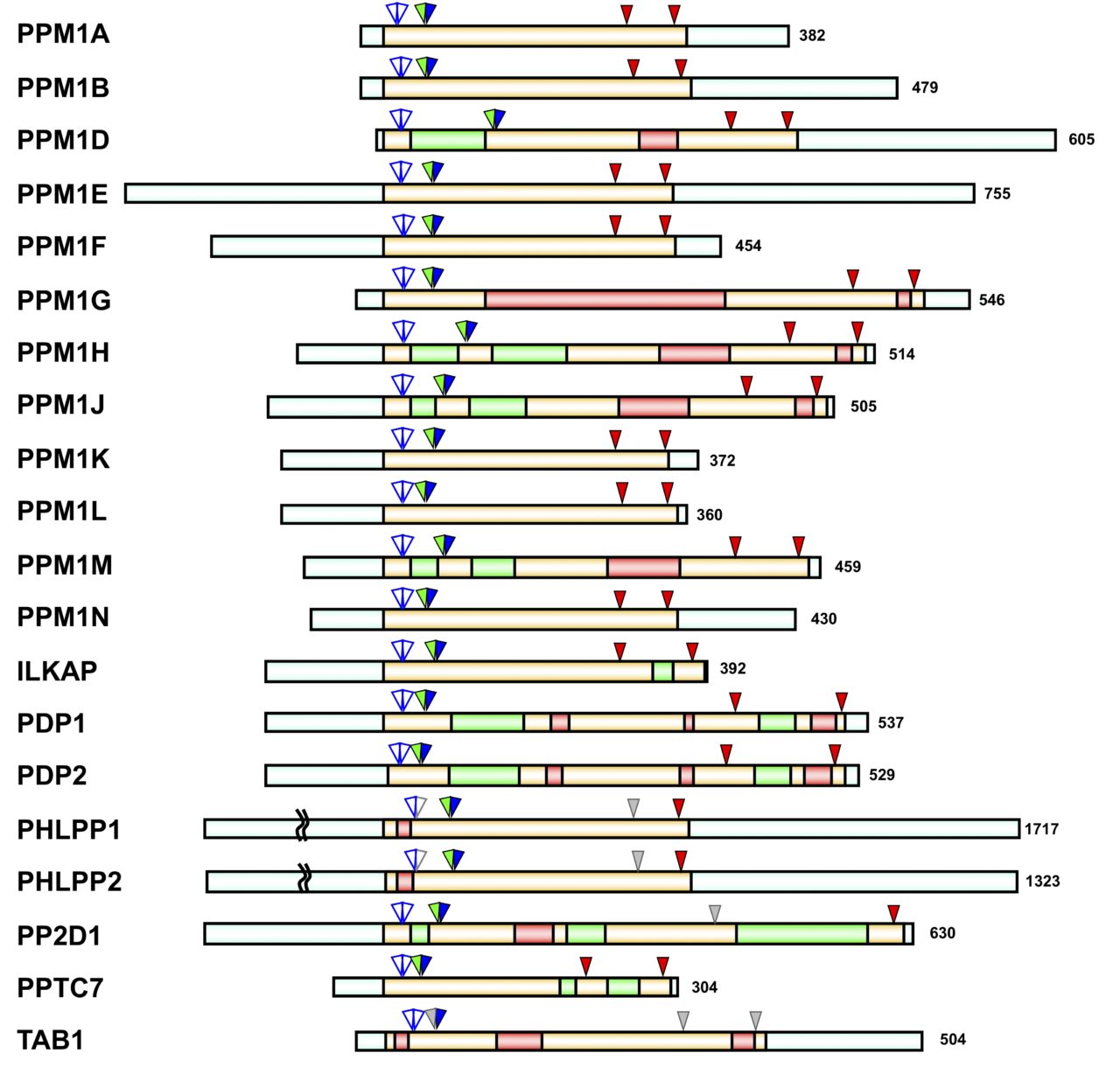
The PPM family regulates stress response, apoptosis, and the cell cycle through various signal transduction pathways. Dysfunction and enhancement due to genetic anomalies of the PPM family has recently been reported to be involved in cancer, hypotension, and many other diseases. The PPM family is Mn2+/Mg2+ dependent Ser/Thr phosphatase, and there are 20 isoforms in humans. In our laboratory, we are developing a new substrate identification method for PPM1 phosphatase. We are working to clarify the relationship between genetic abnormalities of PPM1 family phosphatases and diseases through the identification of target proteins of each isoform and the development of isoform specific inhibitors.
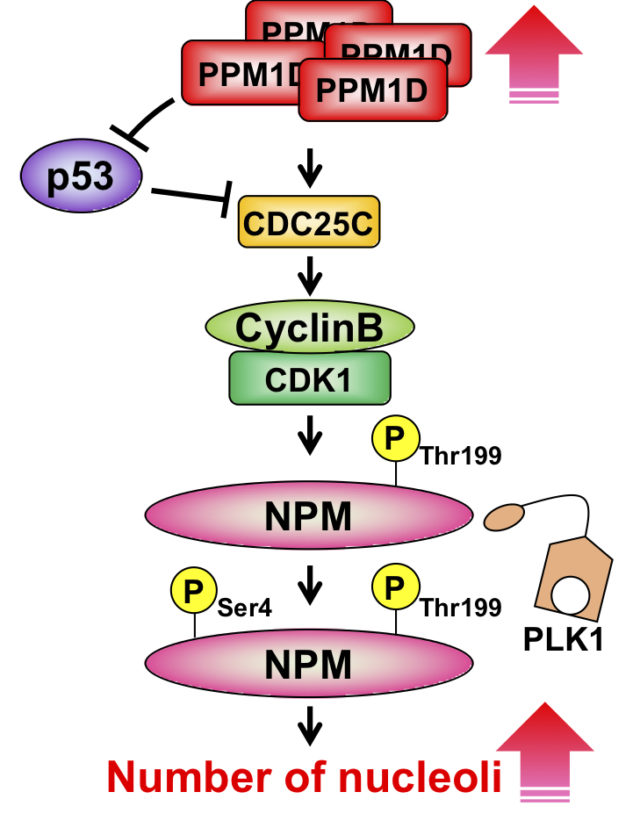
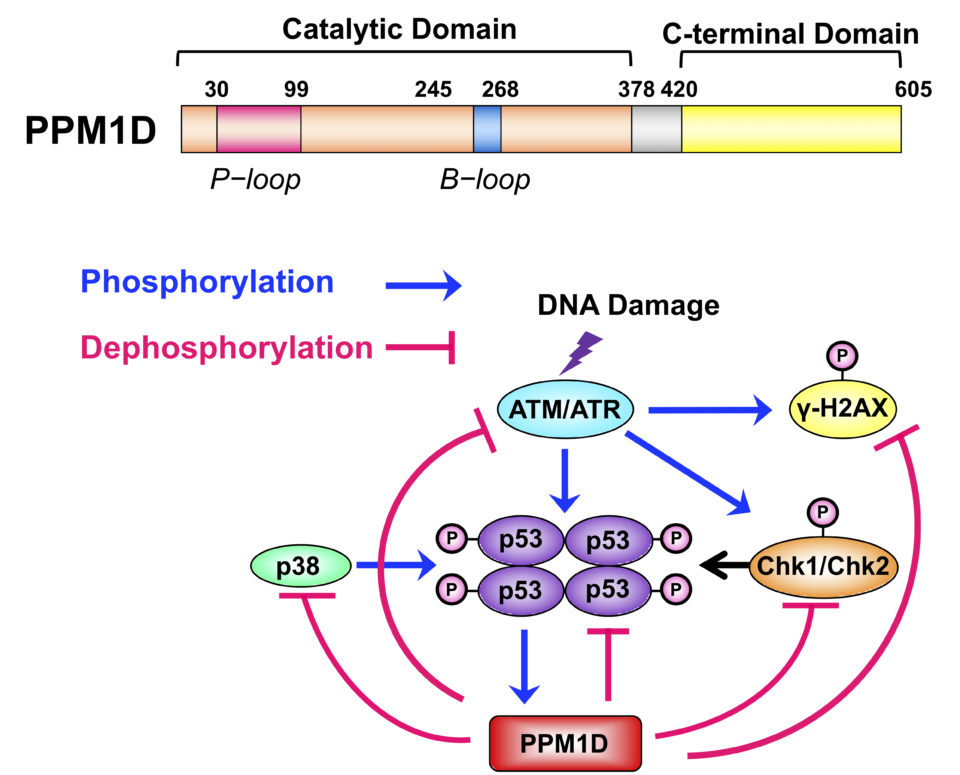
PPM1D (protein phosphatase magnesium-dependent 1, delta), which belongs to the PPM1 type Ser/Thr phosphatases, is induced in a tumor suppressor protein p53-dependent fashion when DNA is damaged by genotoxic stress, such as UV rays or electromagnetic radiation. In normal cells, PPM1D dephosphorylates and deactivates the p53, p38, and ATM proteins, which are involved in cell cycle arrest and apoptosis activated in association with DNA damage; PPM1D is thus considered to return the cell cycle to its original state (Fig. 1). On the other hand, PPM1D gene amplification is observed in breast cancer (15%) and many other forms of cancer. Additionally, because PPM1D knockout mice possess tumorigenesis resistance, PPM1D overexpression is considered to be strongly involved in oncogenesis. However, much is unknown regarding the mechanism of tumorigenesis by PPM1D overexpression. Understanding this mechanism is of paramount importance in cancer therapy that targets PPM1D.
In our laboratory, we are working to elucidate the mechanism of tumorigenesis by PPM1D overexpression through identification of novel binding proteins and intracellular localized analysis of PPM1D. We have reported that overexpression of PPM1D increased the number of nucleoli, which are used as indicators of cancer cytology. The nucleolus is a membraneless organelles, which is a field of ribosome biogenesis formed via the liquid-liquid phase separation. We are working to understand the regulation of mechanism of nucleolar formation via the nucleolar protein NPM and PPM1D.
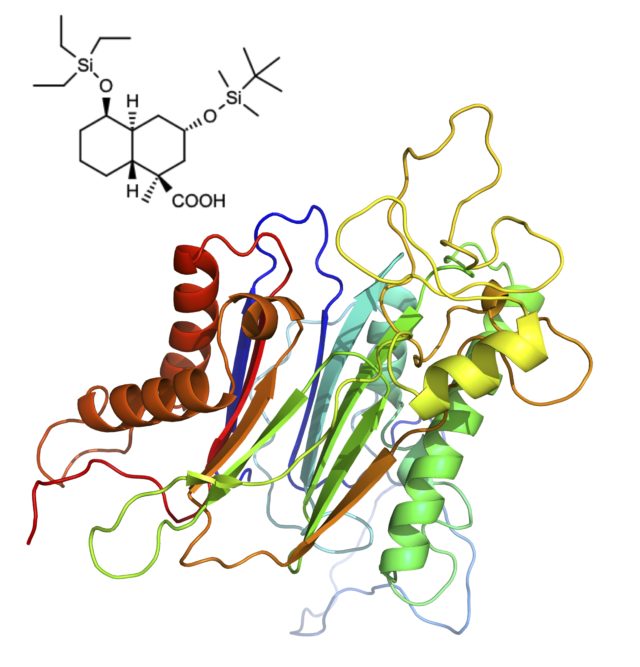
Moreover, PPM1D inhibitors show promise as novel anti-cancer drugs. We have succeeded in developing peptidic inhibitors and small molecule inhibitors, “SL-inhibitors”, with strong inhibition activity against PPM1D. The “SL inhibitor” is extremely effective in vitro and in vivo, so it is expected as a molecular-targeted cancer drug.