【第34回】Ice-Binding Proteins from Polar Microbes, Yeast and Microalgae: Types, Functions, and Application
講演者
Dr. Hak Jun Kim
(Department of Chemistry, Pukyong National University, Busan, Korea)
日時
平成26年8月6日(水) 14:00-15:00
場所
北海道大学理学部 6号館 6-1-03室
概要
Ice-binding proteins (IBPs) are, literally, referred to a group of proteins that bind to ice crystals. IBPs encompass antifreeze proteins (AFPs), ice nucleation proteins (INPs), ice recrystallization protein, and ice anchoring protein and so on. In most cases IBPs and AFPs are interchangeable terms. IBPs, although structurally diverse, bind to the surface of ice crystals and control the growth of ice crystal growth. This binding causes difference of melting and freezing points, termed thermal hysteresis (TH)
and ice recrystallization inhibition (RI). Extensive studies have shown that IBPs from fish, insects, and plants are very effective in ice RI. This ability seems to protect membranes from freezing injury and thus to help the organisms survive at extremely cold environments.
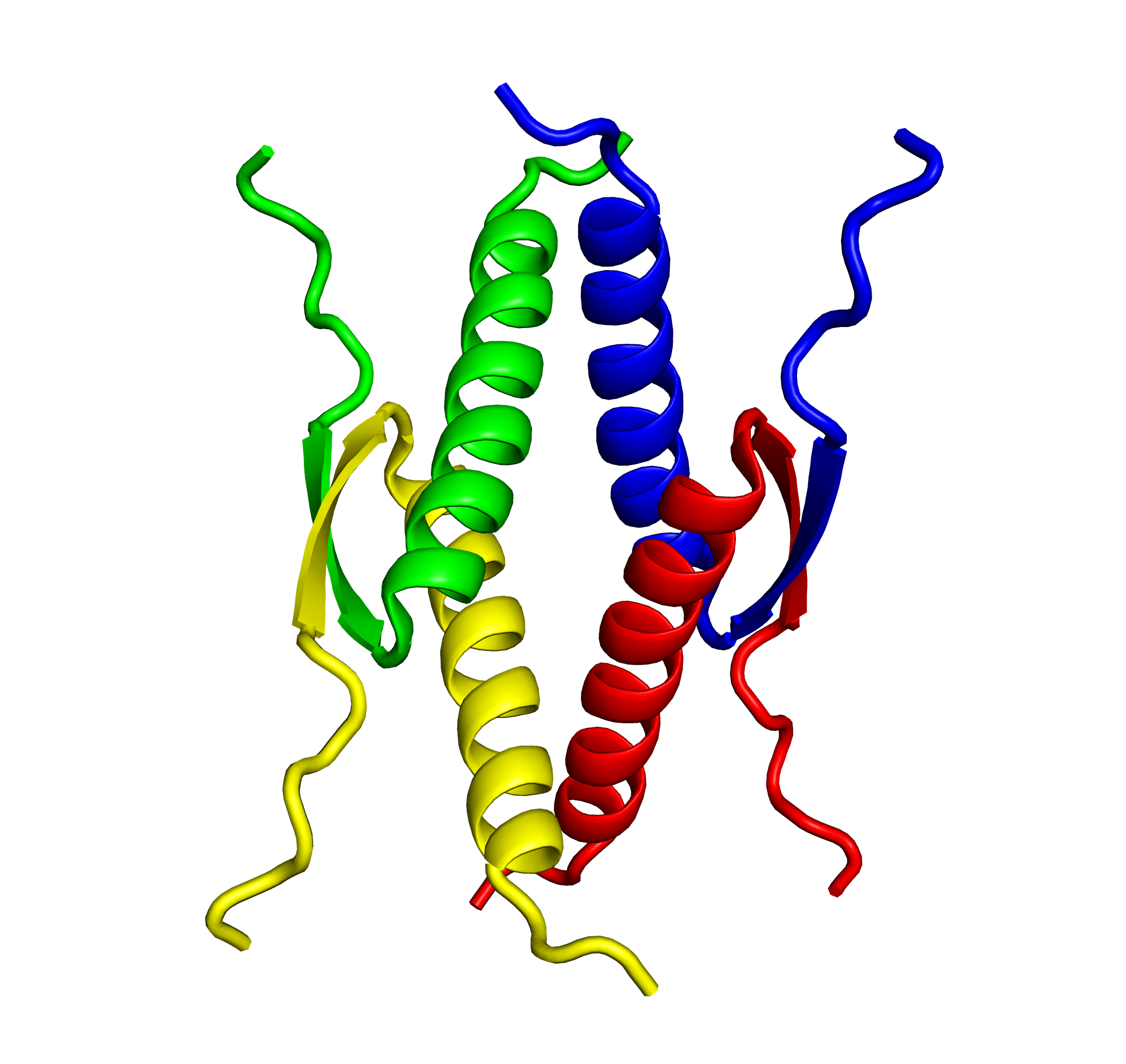
We recently identified IBP from the Antarctic bacterium Flavobacterium frigoris PS1 colonized in sea ice together with sea ice microalgae. The X-ray crystal structure of FfIBP was determined to 2.1 Å resolution to gain insight into its ice binding mechanism. The refined structure of FfIBP shows an intramolecular disulfide bond, and analytical ultracentrifugation and analytical size-exclusion chromatography show that it behaves as a monomer in solution. Although FfIBP closely resembles previously characterized Leucosporidium (recently re-classified as Glaciozyma) IBP (LeIBP) in its amino-acid sequence, the
thermal hysteresis (TH) activity of FfIBP appears to be tenfold higher than that of LeIBP.
Here we present characterization, structural determination, and application of marine bacterial IBP and its comparison with closely related LeIBP.
主催
生命分子化学セミナー
共催
日本生化学会北海道支部
連絡先
北海道大学 大学院理学研究院 化学部門
生物化学研究室
坂口 和靖
TEL: 011-706-2698