Gold nanocatalysis
Reactivity of free clusters
We focus our research on theoretical investigation of unusual catalytic properties of gold clusters for oxidation reactions by molecular oxygen. We have demonstrated that catalytic activation of the adsorbed O2 on small pure gold clusters cannot lead to its dissociation. However, coadsorption of simple hydrocarbons, such as ethylene, C2H4, results in extra charge transfer from the gold cluster to O2, energetically promoting oxygen dissociation. Therefore, O2 dissociation on the surface of small gold clusters is sensitive to the presence of other adsorbates, including the reactant molecule itself. This effect can be particularly important for understanding the mechanism of catalytic oxidation on gold clusters. We have also found an effect of the cooperative adsorption of O2 and C2H4 on small gold clusters. This finding indicates that the process of oxygen dissociation on the surface of gold clusters is sensitive to the presence of other adsorbates, including the reactant molecule itself.
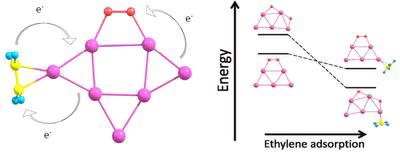
Reactant promoted oxygen dissociation.
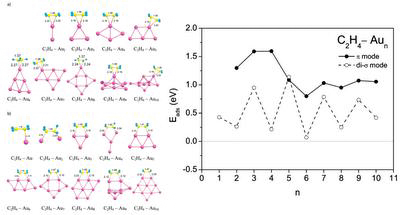
Adsorption of ethylene molecules on neutral, anionic, and cationic gold clusters demonstrates very interesting features. Thus, C2H4 can be adsorbed on small gold clusters in two different configurations, corresponding to the π- and di-σ-bonded species. A striking difference is found in the size dependence of the adsorption energy of C2H4 bonded to the neutral gold clusters in the π and di-σ configurations. The electronic shell effects play an important role for the di-σ mode of ethylene adsorption on neutral gold clusters.
Reactivity of supported clusters
Support effect is one of the most important factors in nanocatalysis. We have demonstrated that hexagonal boron nitride (h-BN) surface which was traditionally considered as an inert support can strongly modify properties of the gold clusters and act as an “active” support. In particular, the structural, electronic, and catalytic properties of Au and Au2 supported on the pristine and defected h-BN surface have been studied. It is demonstrated that adsorption and catalytic activation of O2 on the h-BN supported Au and Au2 can be affected by the interaction with the support via electron pushing and donor/acceptor mechanisms.
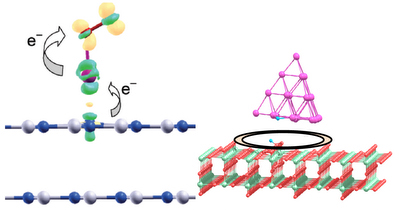
Effects of inert and active supports on catalytic activity of gold.
Adsorption and dissociation of H2 on gold clusters supported on the active rutile TiO2(110) surface has been studied. Rutile TiO2(110) support energetically promotes H2 dissociation on gold clusters. The perimeter interface between gold nanoparticle and TiO2 surface acts as an active site for H2 dissociation. Performed calculations can explain recent experimental findings of T.Fujitani et al, Angew. Chem. Int. Ed., 48, 9515 (2009).
Relevant publications
- M. Gao, A. Lyalin, and T. Taketsugu, CO oxidation on h-BN supported Au atom, J. Chem. Phys. 138, 034701 (2013).
- M. Gao, A. Lyalin, and T. Taketsugu, Oxygen activation and dissociation on h-BN supported Au atoms, Int. J. Quantum Chem. 113, 443-452 (2013).
- M. Gao, A. Lyalin, and T. Taketsugu, Catalytic activity of Au and Au2 on h-BN surface: adsorption and activation of O2, J. Phys. Chem. C 116, 9054–9062, (2012).
- M. Gao, A. Lyalin, and T. Taketsugu, Role of the support effects on the catalytic activity of gold clusters: a density functional theory study, Catalysts 1, 18-39, (2011).
- A. Lyalin and T. Taketsugu, A computational investigation of H2 adsorption and dissociation on Au nanoparticles supported on TiO2 surface, Faraday Discussions 152, 185-201, (2011).
- A. Lyalin and T. Taketsugu, Reactant promoted oxygen dissociation on gold clusters, J. Phys. Chem. Lett. 1, 1752-1757, (2010).
- A. Lyalin and T. Taketsugu, Adsorption of ethylene on neutral, anionic and cationic gold clusters, J. Phys. Chem. C 114, 2484-2493, (2010).
- A. Lyalin and T. Taketsugu, Cooperative adsorption of O2 and C2H4 on small gold clusters, J. Phys. Chem. C 113, 12930-12934, (2009).



