発表論文
2026
214. 化学産業の無人化に向けた自動合成装置の開発
芦刈洋祐、永木愛一郎
MATERIAL STAGE 2026, 25 (11), 47–52.
(技術情報協会マテリアルステージ2026年2月号)
Link

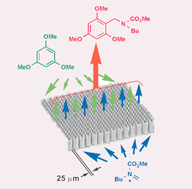
213. Crystallization-Based Approach To Continuous Manufacturing: A Case Study of Acetaminophen Production
Masuda, K.; Yamamoto, T.; Kobayashi, K.; Sano, H.; Saito, I.; Nagaki, A.; Yada, A.
Org. Process Res. Dev. 2026, in press.
DOI: 10.1021/acs.oprd.5c00332
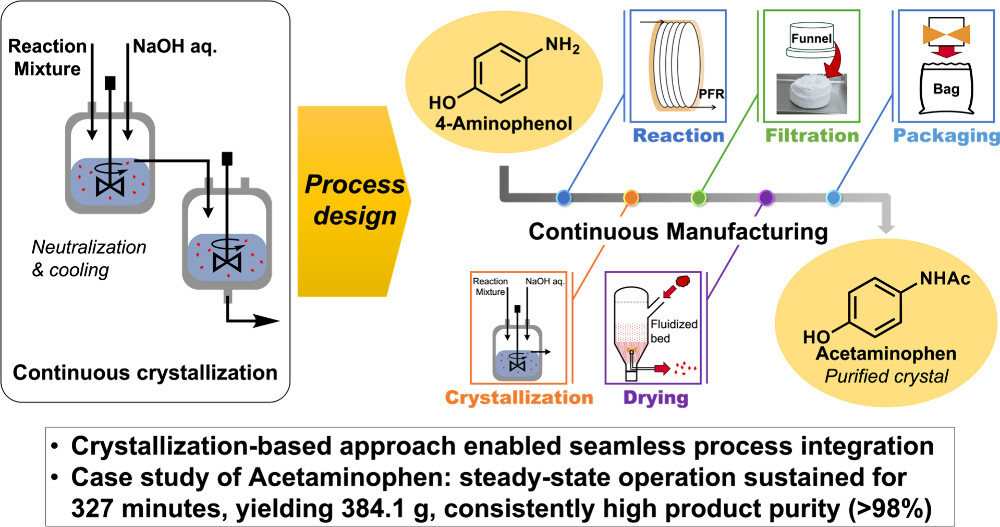
XX. Sequence-controlled Rapid Living Anionic Copolymerization of Polystyrene Derivatives Using a Flow Microreactor
Miyagishi, H. V.; Oshida, S.; Sakashita, K.; Sakaue, H.; Nagaki, A.
ChemRxiv 2026.
DOI: 10.26434/chemrxiv-2026-2p8xf
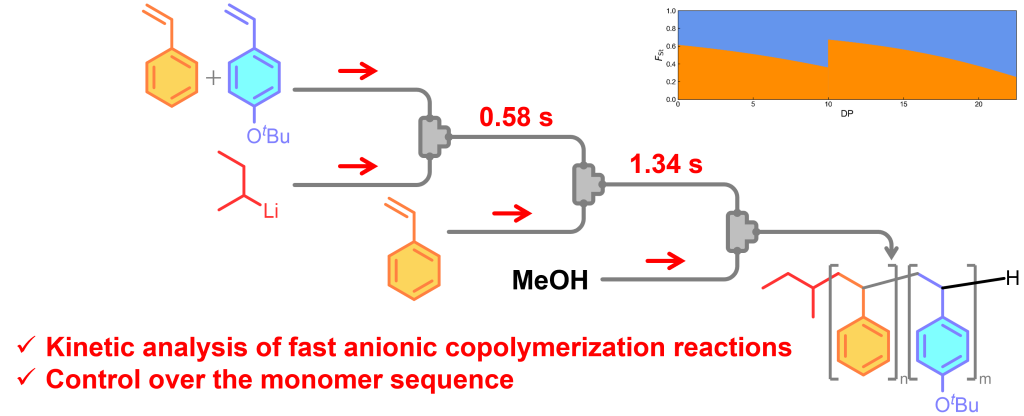
212. Reductive Generation of Anionic C1 Carbenoid Species Attached to Organosulfur Functional Groups
Okamoto, K.; Wada, S.; Muta, K.; Tsuchihashi, Y.; Nagaki, A.
Chem. Asian J. 2026, 21, e70525.
DOI: 10.1002/asia.70525
2025
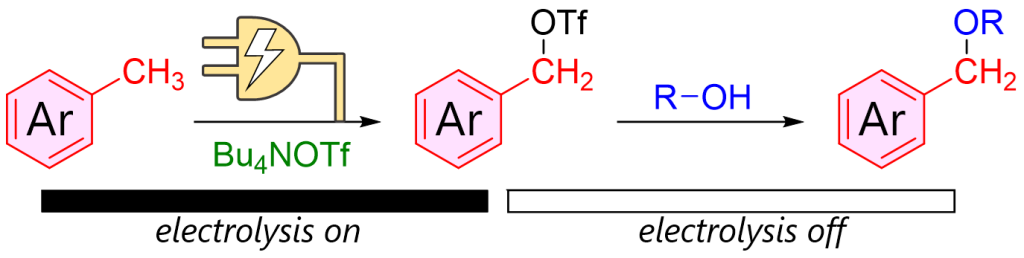
211. Generation and Reaction of Benzyl Triflates by Anodic Oxidation of Toluenes
Ashikari, Y.; Kudo, T.; Nagaki, A.
Electrochemistry 2025, 93, 127001.
DOI: 10.5796/electrochemistry.25-00142
Selected as Editor’s choice!
https://doi.org/10.5796/electrochemistry.25-00142cover
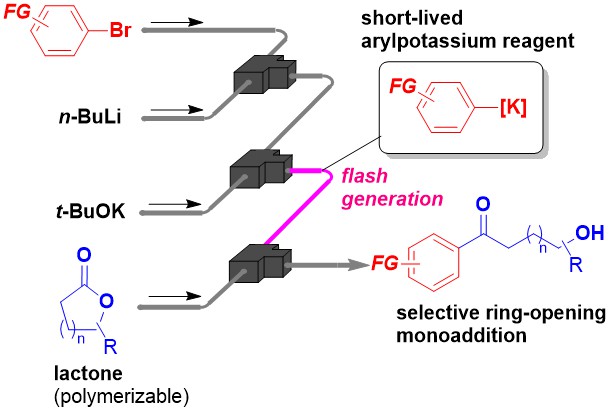
210. Flash-Flow Generation and Selective Monoaddition of Organopotassium Species to Lactones
Yoo, D.-E.; Okamoto, K.; Takeda, M.; Nakayama, H.; Ebisawa, R.; Nagaki, A.
ChemSusChem 2025, 19, e202502201.
DOI: 10.1002/cssc.202502201
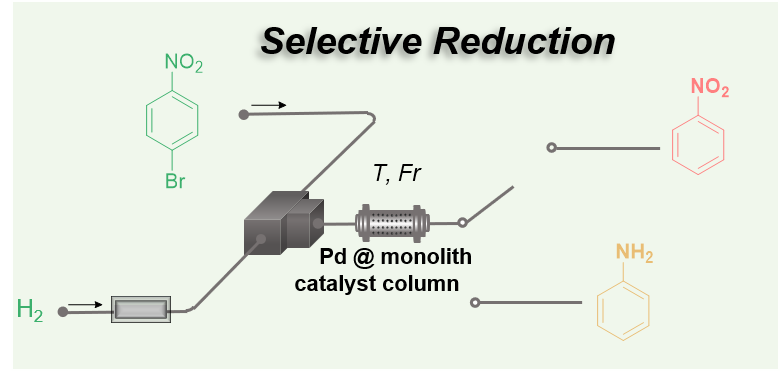
209. Chemoselective Reduction of Aryl Halide Bearing Nitro Group Enabled by Heterogeneous Monolith Supported Pd Catalyst
Zhong, X.; Tsuchihashi, Y.; Yamamoto, Z.; Okamoto, K.; Nagaki, A.
Top. Catal. 2025.
DOI: 10.1007/s11244-025-02242-5
208. 重合の高度制御を基軸とするシークエンスオリゴマー合成への展開
永木愛一郎、岡本和紘
高分子 2025, 74 (11), 609–611.

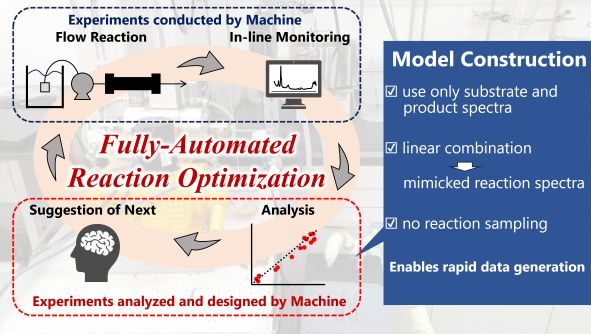
207. Real-time inline-IR-analysis via linear-combination strategy and machine learning for automated reaction optimization
Ashikari, Y.; Tamaki, T.; Tomite, K.; Yonekura, Y.; Nagaki, A.
Commun. Chem. 2025, 8, 287.
DOI: 10.1038/s42004-025-01676-y
ChemRxiv
DOI: 10.26434/chemrxiv-2024-vtdv1
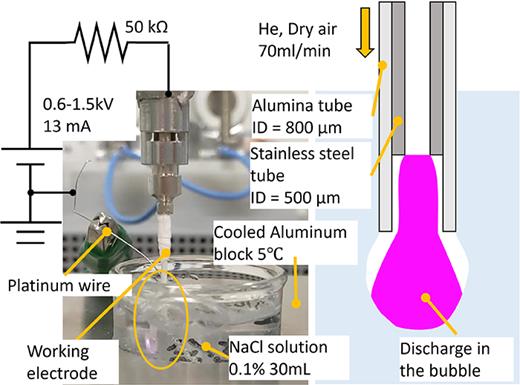
206. One-step conversion of benzene to phenol in plasma-irradiated benzene solution
Inagaki, Y.; Hayashi, Y. R.; Sasaki, K.; Takakusagi, S.; Okamoto, K.; Nagaki, A.; Shirai, N.
J. Appl. Phys. 2025, 138, 113301.
DOI: 10.1063/5.0282963
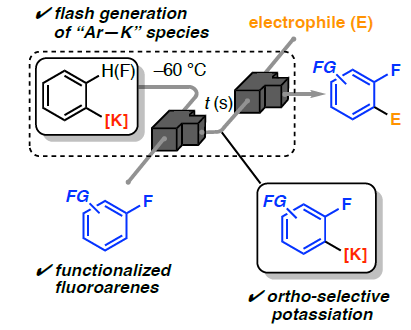
XX. Disguised Reactivities of Arylpotassium Species for Chemoselective Deprotonation in Microflow Reaction Field
Okamoto, K.; Nakayama, H.; Kamio, S.; Nagaki, A.
ChemRxiv
DOI: 10.26434/chemrxiv-2025-c2w7r
205. Fast Oxidative Coupling Giving Functionalized Biaryls via Organocuprates
Okamoto, K.; Ebisawa, R.; Nakayama, H.; Nagaki, A.
Eur. J. Org. Chem. 2025, 28, e202500072.
DOI: 10.1002/ejoc.202500072

XX. Sub-Millisecond Mixing in a T-Mixer in the Turbulent Flow Regime: Quantitative Chemiluminescence-Based Analysis
Ishii, Y.; Ogawa, T.; Onda, K.; Miyagishi, H. V.; Ashikari, Y.; Nagaki, A.; Asano, S.; Miyata, K.
ChemRxiv
DOI: 10.26434/chemrxiv-2025-llskv

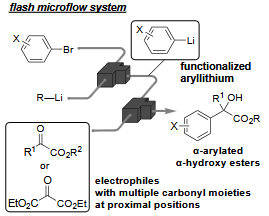
204. Efficient and Selective Transformations of α-Keto Esters into α-Arylated α-Hydroxy Esters Using Continuous-Flow System
Qenawy, M. S.; Okamoto, K.; Nagaki, A.
Chem. Eur. J. 2025, 31, e202500299.
DOI: 10.1002/chem.202500299
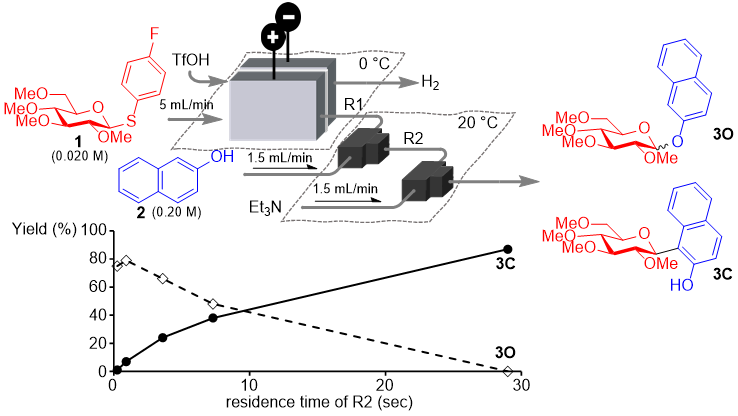
203. O–C Glycoside Rearrangement through Time-Controlled Electrochemical Flow Strategy: Switching between Kinetics and Thermodynamics
Ashikari, Y.; Yao, Y.; Kudo, T.; Takumi, M.; Nagaki, A.
Eur. J. Org. Chem. 2025, 28, e202500038.
DOI: 10.1002/ejoc.202500038
Selected as a Front Cover Picture!
DOI: 10.1002/ejoc.202581901
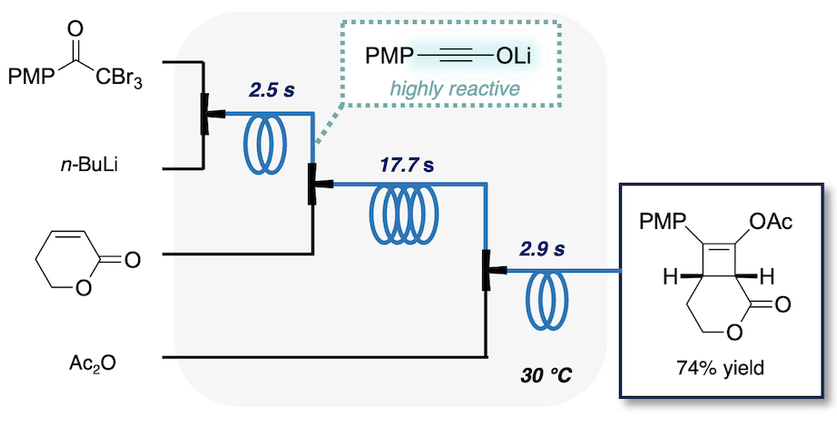
202. Continuous flow synthesis of cyclobutenes via lithium ynolates
Koyama, A.; Namioka, M.; Naka, H.; Ashikari, Y.; Nagaki, A.; Takikawa, H.; Yamaoka, Y.; Takasu, K.
Green Chem. 2025, 27, 2760–2765.
DOI: 10.1039/D4GC05102E
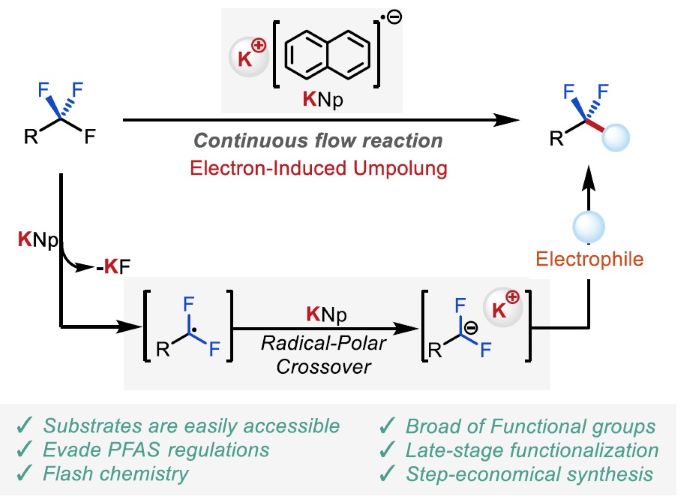
201. Defluorinative Functionalization Approach led by Difluoromethyl Anion Chemistry
Muta, K.; Okamoto, K.; Nakayama, H.; Wada, S.; Nagaki, A.
Nat. Commun. 2025, 16, 416.
DOI: 10.1038/s41467-024-52842-0
ChemRxiv
DOI: 10.26434/chemrxiv-2024-5ml66-v3
北海道大学プレスリリース
「フロー法による炭素-フッ素結合の直接変換に成功~創薬研究・開発プロセスの迅速化に期待~」
200. Anionic polymerization Driven by Flowmicro Chemistry
Nagaki, A. Ashikari, Y.
Polymer J. 2025, 57, 143–148.
DOI: 10.1038/s41428-024-00972-z
Selected as a Cover Picture.
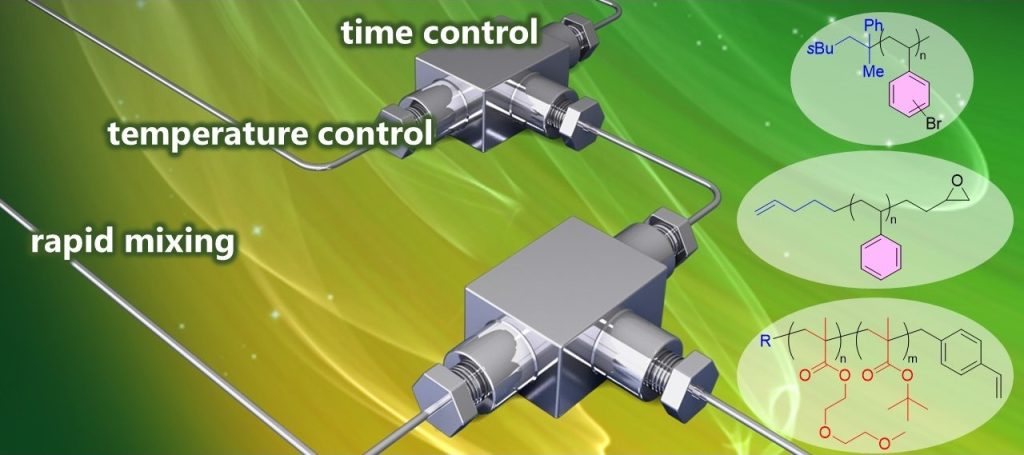

199. Use of Organolithiums in Flow Chemistry (8.1.35)
Miyagishi, H. V.; Nagaki, A.
Science of Synthesis, Knowledge Updates 2025/1
Georg Thieme Verlag KG: Stuttgart, 2025.
DOI: 10.1055/sos-sd-108-00434

198. フロー合成におけるベイズ最適化の活用と自動合成(第6章4節)
芦刈洋祐、永木愛一郎
ベイズ最適化の活用事例 材料探索/物性予測/配合・プロセス条件の最適化
技術情報協会、2025年3月31日発刊
ISBN: 978-4-86798-066-8. LINK

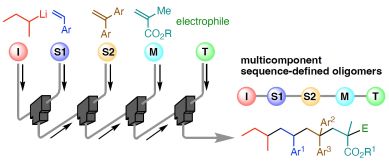
197. Sequence-Defined Synthesis Enabled by Fast and Living Anionic Monoaddition of Vinyl Monomers
Okamoto, K.; Yoo, D. E.; Yoshioka, R.; Nakasato, R.; Ashikari, Y.; Kitayama, K.; Nagaki, A.
Angew. Chem. Int. Ed. 2025, 64, e202416875.
DOI: 10.1002/anie.202416875
ChemRXiv
DOI: 10.26434/chemrxiv-2024-jffkv-v2
2024
196. フロー合成によるファインケミカル品の生産(第5章8節)
宮岸拓路、永木愛一郎
195. 連続フロー法による有機合成とその事例(第1章12節)
岡本和紘、永木愛一郎
194. フロー合成における自動化・ロボット化技術とその活用法(第1章6節)
芦刈洋祐、永木愛一郎
ファインケミカル,医薬品の連続生産プロセス -フロー合成/分離・精製/粉体プロセス/モニタリング-
技術情報協会、2024年11月29日発刊
ISBN: 978-4-86798-051-4. LINK

193. ありのままの自分で新しい扉を(Message from Young Principal Researcher (MyPR))
永木愛一郎
有機合成化学協会誌 2024, 82 (10), 1021–1023.
DOI: 10.5059/yukigoseikyokaishi.82.1021
オープンアクセス

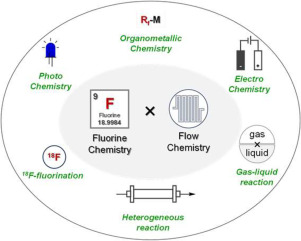
192. Recent Advances in Fluorine Chemistry using Flow Technology
Muta, K.; Soutome, H.; Nagaki, A.
J. Fluorine Chem. 2024, 279, 110349.
DOI: 10.1016/j.jfluchem.2024.110349
191. 連続プロセス構築への機械学習の活用~フロー合成の事例(第8章第9節)
早乙女広樹、永木愛一郎
最新GMPおよび関連ICHガイドライン対応実務
技術情報協会、2024年9月30日発刊
ISBN: 978-4-86798-041-5. LINK

190. 有機リチウム化合物発生におけるフロー選択性制御と反応開発
岡本和紘、永木愛一郎
189. AIを活用したフロー化学研究
芦刈洋祐、永木愛一郎
月刊ファインケミカル2024年7月号「フロー・マイクロ合成―次世代合成の最前線―」
シーエムシー出版、2024年7月15日発刊
ISSN: 0913-6150. LINK

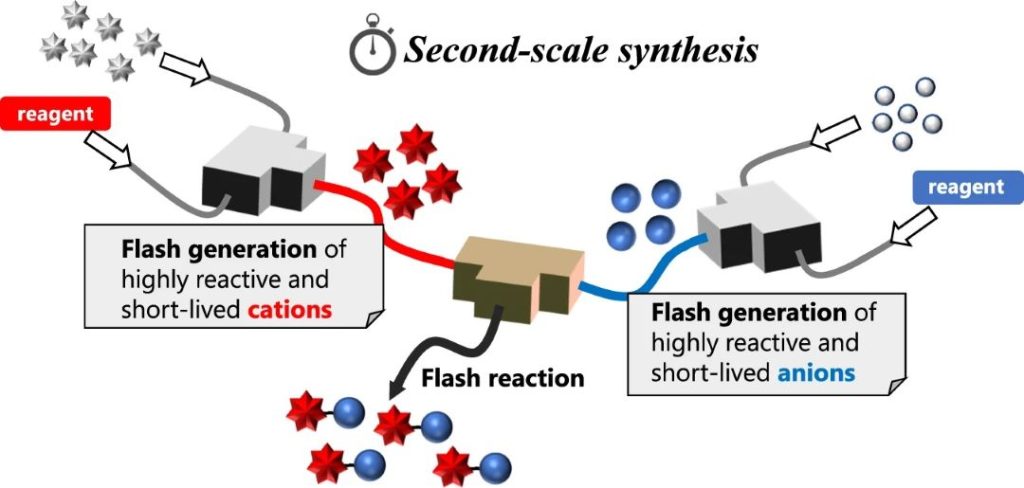
188. Convergent approach for direct cross-coupling enabled by flash irreversible generation of cationic and anionic species
Soutome, H.; Yamashita, H.; Shimizu, Y.; Takumi, M.; Ashikari, Y.; Nagaki, A.
Nat. Commun. 2024, 15, 4873.
DOI: 10.1038/s41467-024-48723-1
北海道大学プレスリリース
「フロー法によりカチオンとアニオンの直接反応に成功~刹那の活性種発生による超高速な化学反応~」
187. 連続プロセス構築への機械学習の活用~フロー合成の事例(第4章第1節第2項)
早乙女広樹、永木愛一郎
プロセスインフォマティクス~データサイエンスで変わる材料開発と製造~
情報機構、2024年6月19日発刊
ISBN:978-4-86502-270-4. Link

186. Expanding the Scope of C-Glycoside Synthesis from Unstable Organolithium Reagents Using Flow Microreactors
Miyagishi, H. V.; Kimuro, Y.; Ashikari, Y.; Nagaki, A.
Org. Lett. 2024, 26, 5032–5036.
DOI: 10.1021/acs.orglett.4c01698
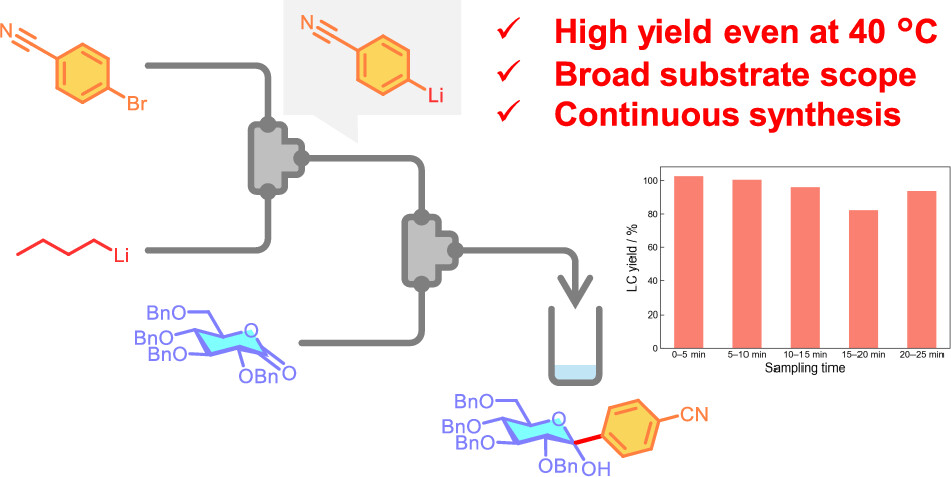
185. Flow Chemistry of Metal Carbenoid Species towards Selective Organic Synthesis
Okamoto, K.; Nagaki, A.
Synthesis 2024, 56, 2899–2908.
Special issue Flow Chemistry
DOI: 10.1055/a-2302-5363
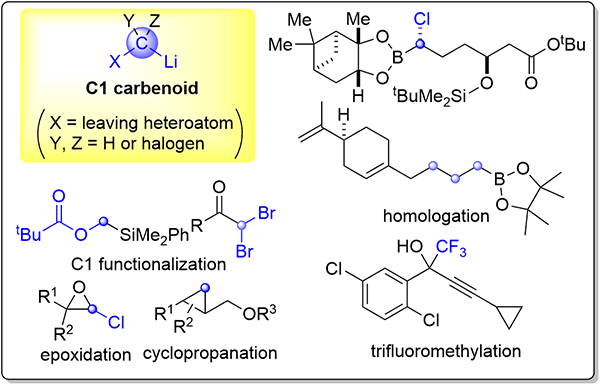
184. Spatiotemporal Control of Protein Refolding through Flash-Change Reaction Conditions
Nakahara, Y.; Kawaguchi, T.; Matsuda, Y.; Endo, Y.; Date, M.; Takahashi, K.; Kato, K.; Okasora, T.; Ejima, D.; Nagaki, A.
Langmuir 2024, 40, 8483–8492.
DOI: 10.1021/acs.langmuir.4c00024
183. Flowmicro In-Line Analysis-Driven Design of Reactions mediated by Unstable Intermediates: Flash Monitoring Approach
Ashikari, Y.; Yoshioka, R.; Yonekura, Y.; Yoo, D. E.; Okamoto, K.; Nagaki, A.
Chem. Eur. J. 2024, 30, e202303774.
DOI: 10.1002/chem.202303774
Selected as a Front Cover Picture.
DOI: 10.1002/chem.202400844
Cover profile
北海道大学プレスリリース
「70年の時を経てアニオン重合反応の活性種観測に成功~精密な高分子材料合成への貢献に期待~」
日刊工業新聞(2024年3月14日)
「北大、アニオン重合の反応制御 高分子材開発に知見提供」

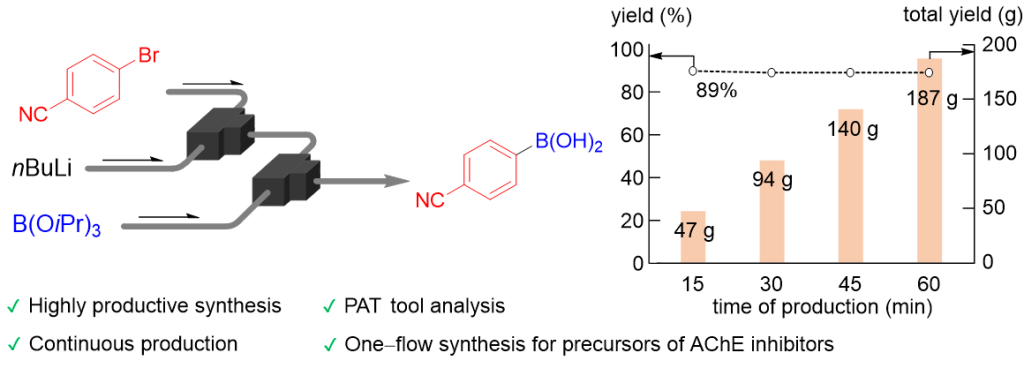
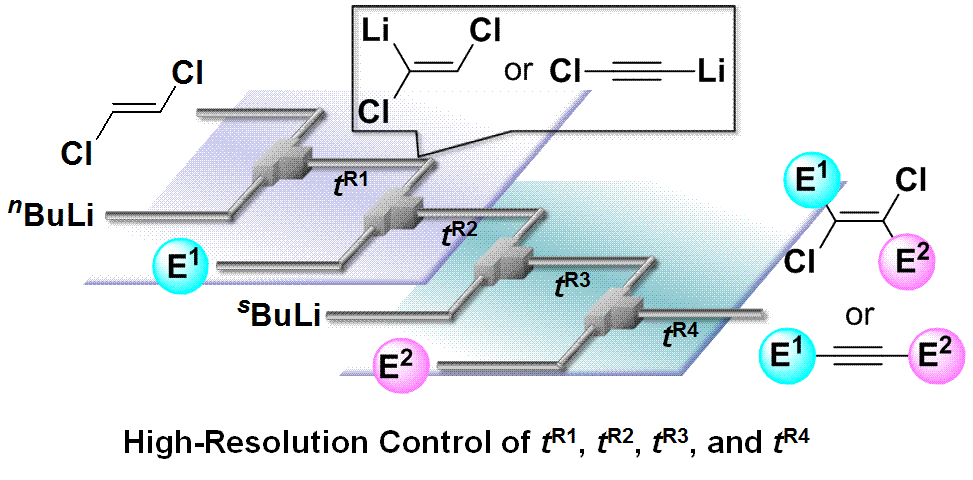
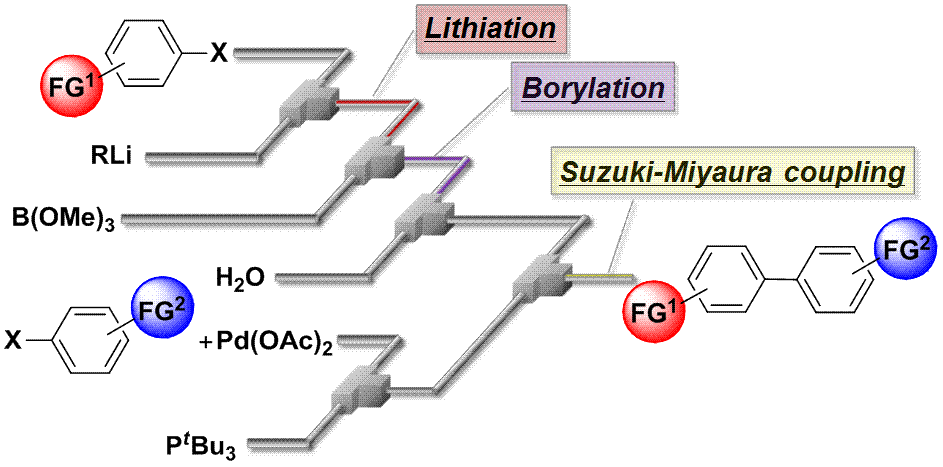
182. Highly productive flow synthesis for lithiation, borylation, and/or Suzuki coupling reaction
Soutome, H.; Maekawa, K.; Ashikari, Y.; Nagaki, A.
Org. Process Res. Dev. 2024, 28, 2006–2012.
DOI: 10.1021/acs.oprd.4c00021
Selected as a Cover Picture

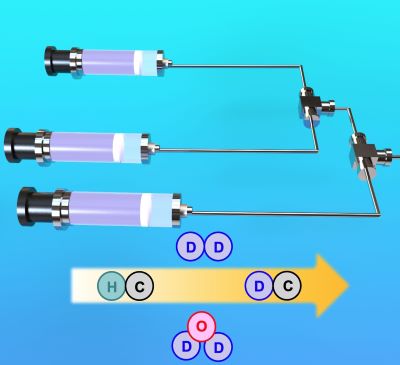
181. Synthesis of Deuterated Compounds by Flow Chemistry
Kamio, S.; Okamoto, K.; Yamagishi, T.; Nagaki, A.
ChemPlusChem 2024, 89, e202300744.
DOI: 10.1002/cplu.202300744
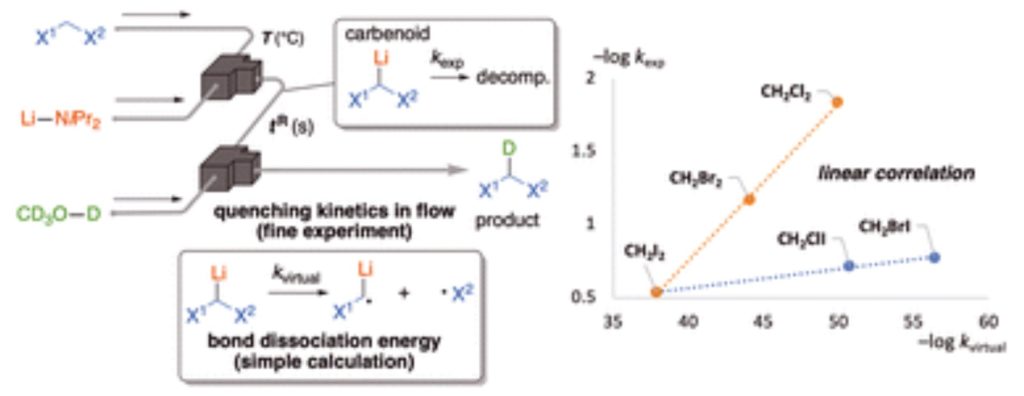
180. Elucidation of the kinetic stabilities of carbenoid species by integration of theoretical and experimental studies
Okamoto, K.; Muta, K.; Yamada, H.; Higuma, R.; Ashikari, Y.; Nagaki, A.
React. Chem. Eng. 2024, 9, 1173–1178.
DOI: 10.1039/D3RE00648D
179. リビング重合の制御性向上に寄与するフローマイクロリアクターシステム(第2章第4節)
早乙女広樹、芦刈洋祐、永木愛一郎
リビング重合技術 高度な制御を可能にする精密重合と応用展開
サイエンス&テクノロジー、2024年10月29日発刊
ISBN: 978-4-86428-322-9. Link

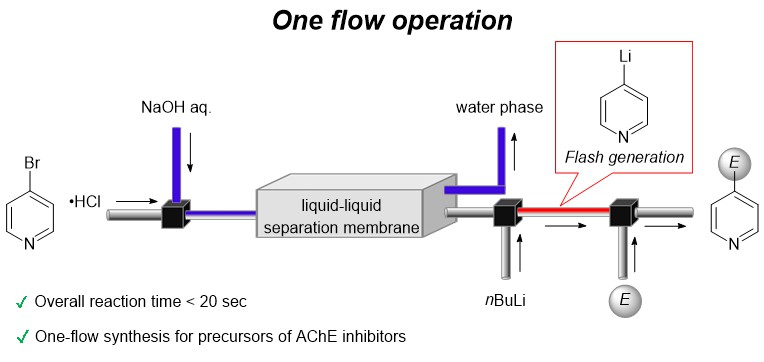
178. One-flow operation via 4-bromopyridine enables flash synthesis of AChE inhibitor
Soutome, H.; Kimuro, Y.; Kawaguchi, T.; Yoo, D. E.; Yao, Y.; Oshida, S.; Nakayama, H.; Iwata, M.; Ebisawa, R.; Kikuchi, R.; Tomite, K.; Wada, S.; Ashikari, Y.; Nagaki, A.
Synthesis 2024, 56, 821–827.
DOI: 10.1055/a-2218-9048
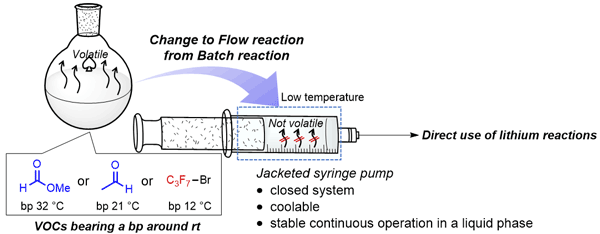
177. Reactions of Highly Volatile Organic Compounds with Organolithium Species in Flow Microreactor
Muta, K.; Okamoto, K.; Nagaki, A.
Synlett 2024, 35, 1259–1264.
DOI: 10.1055/a-2170-2976
176. マイクロリアクターの特長を活かした環境調和型の精密高速合成化学
永木愛一郎
有機合成化学協会誌 2024, 82, 2–13.
DOI: 10.5059/yukigoseikyokaishi.82.2

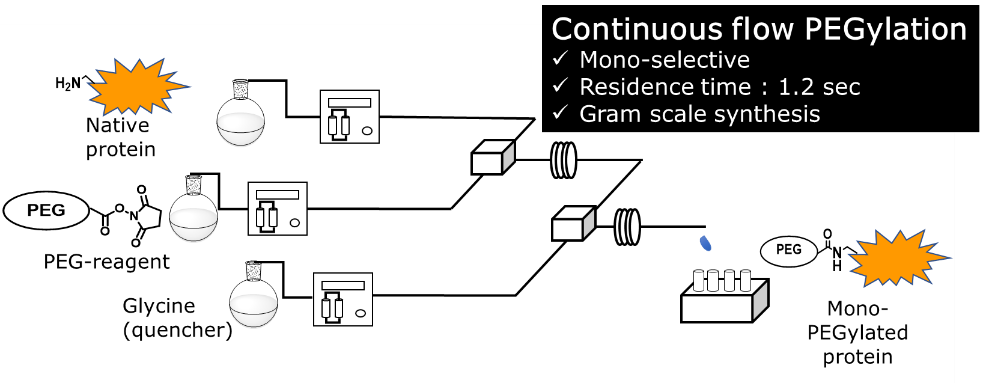
175. A Manufacturing Strategy Utilizing a Continuous Mode Reactor toward Homogeneous PEGylated Bioconjugate Production
Nakahara, Y.; Endo, Y.; Takahashi, K.; Kawaguchi, T.; Kato, K.; Matsuda, Y.; Nagaki, A.
Synthesis 2024, 56, 597–602.
DOI: 10.1055/a-2077-6187
2023
174. AI技術を用いたフロー自動合成と実験の短縮(第7章3節)
芦刈洋祐、玉木孝、永木愛一郎
実験の自動化・自律化によるR&Dの効率化と運用方法 -AI、ロボット技術、ChatGPT、MI、ベイズ最適化、秘密計算など-
技術情報協会、2023年12月28日発刊
ISBN:978-4-86104-994-1. Link

173. 機械学習を活用したフローリアクター合成のプロセス最適化、自動化
芦刈洋祐、永木愛一郎
MATERIAL STAGE 2023, 23 (9), 41–45.
(技術情報協会マテリアルステージ2023年12月号)
Link

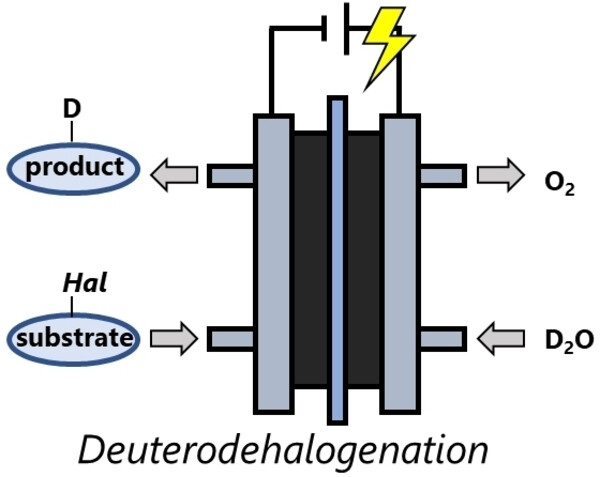
172. Electrocatalytic Reduction of (Hetero)Aryl Halides in a Proton-Exchange Membrane Reactor and its Application for Deuteration
Ashikari, Y.; Mandai, K.; Yao, Y.; Tsuchihashi, Y.; Nagaki, A.
ChemElectroChem 2023, 10, e202300315.
DOI: 10.1002/celc.202300315
Selected as a cover picture.
DOI:10.1002/celc.202300657
171. ベイズ最適化を活用したフロー合成研究事例
玉木孝、岡本和紘、永木愛一郎
PHARMSTAGE 2023, 23 (7), 13–18.
Link

170. フロー研究が導く有機化学の高速化
永木愛一郎、岡本和紘
Organometallic News 2023, 94–99.
DOI: 10.1055/a-2170-2976

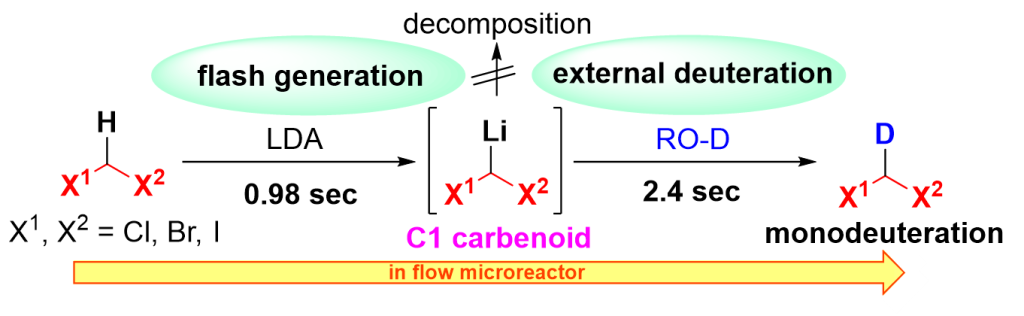
169. External Flash Generation of Carbenoids Enables Monodeuteration of Dihalomethanes
Okamoto, K.; Higuma, R.; Muta, K.; Fukumoto, K.; Tsuchihashi, Y.; Ashikari, Y.; Nagaki, A.
Chem. Eur. J. 2023, 29, e202301738.
DOI: 10.1002/chem.202301738
Selected as a cover picture!
Link

168. 有機電解フロー反応による有用物質の高速合成
宅見正浩、永木愛一郎
ファルマシア 2023, 59 (9), 815–819.
DOI: 10.14894/faruawpsj.59.9_815
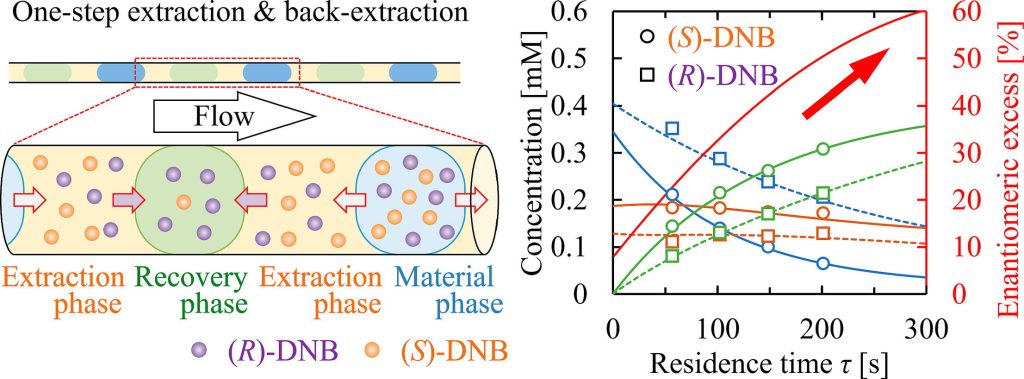
167. Continuous enantiomeric separation using water-oil-water segmented flow system
Muranaka, Y.; Maki, T.; Nakayoshi, D.; Asano, S.; Ikebata, K.; Nagaki, A.; Ashikari, Y.; Mandai, K.; Mae, K.
Chem. Eng. J. 2023, 469, 143891.
DOI: 10.1016/j.cej.2023.143891
166. Introduction to Flow Chemistry for the Synthetic Chemist (chepter 4)
Ashikari, Y.; Nagaki, A.
Enabling Tools and Techniques for Organic Synthesis: A Practical Guide to Experimentation, Automation, and Computation (Ed. Newman, S. G.)
Willy, published in October 2023
ISBN: 978-1-119-85563-7. Link

165. 中間体を自在に操り、高速な有機合成化学を
永木愛一郎
ドラマチック有機合成化学―感動の瞬間100
化学同人、2023年7月20日発刊
ISBN:978-4-7598-2336-3

164. AIによるフロー合成の反応条件最適化(第5章7節)
岡本和紘、永木愛一郎
ケモインフォマティクスにおけるデータ収集の最適化と解析手法
技術情報協会、2023年4月28日発刊
ISBN:978-4-86104-944-6
Link

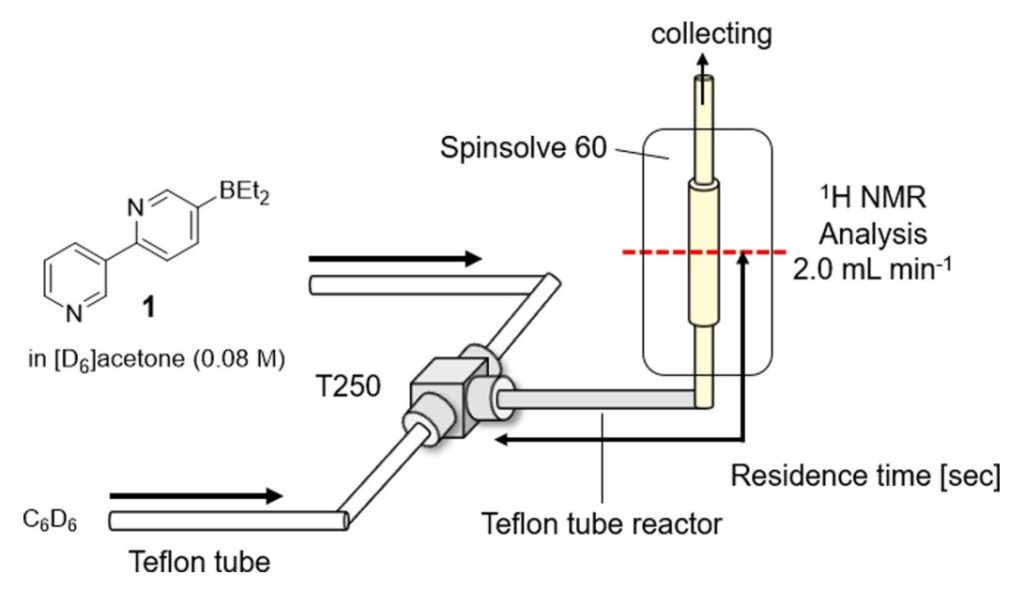
163. Flow-Chemistry-Enabled Synthesis of 5-Diethylboryl-2,3′-bipyridine and Its Self-Assembly Dynamics
Wakabayashi, S.; Takumi, M.; Kamio, S.; Wakioka, M.; Ohki, Y.; Nagaki, A.
Chem. Eur. J. 2023, 29, e202202882.
DOI: 10.1002/chem.202202882
2022
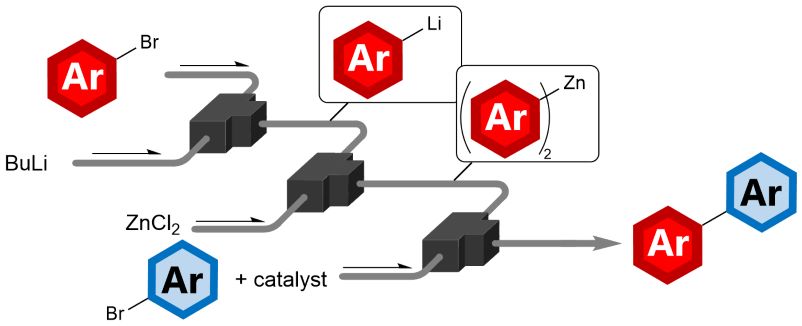
162. Flash Functional Group-Tolerant Biaryl-Synthesis Based on Integration of Lithiation, Zincation and Negishi Coupling in Flow
Ashikari, Y.; Guan, K.; Nagaki, A.
Front. Chem. Eng. 2022, 4, 964767.
DOI: 10.3389/fceng.2022.964767
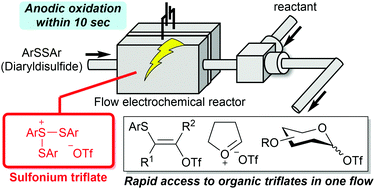
161. Rapid access to organic triflate based on flash generation of unstable sulfonium triflates in flow
Takumi, M.; Sakaue, H.; Shibasaki, D.; Nagaki, A.
Chem. Commun. 2022, 58, 8344–8347.
DOI: 10.1039/D2CC02344J
Selected as a back cover. Link
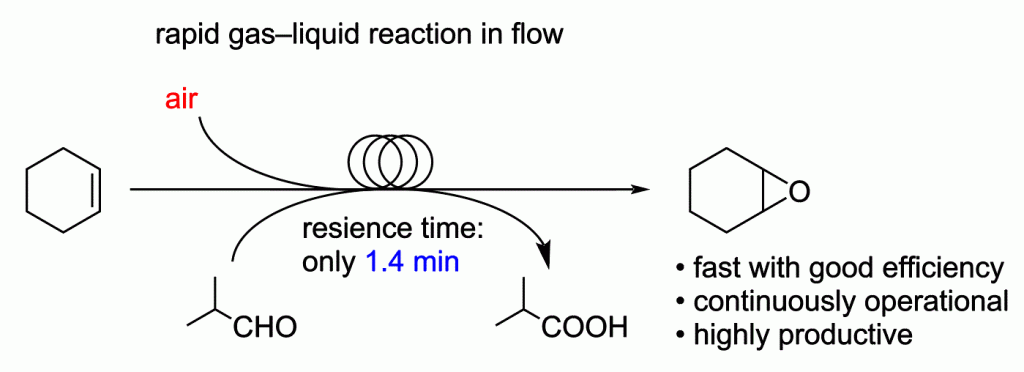
160. Rapid gas-liquid reaction in flow. Continuous synthesis and production of cyclohexene oxide
Mandai, K.; Yamamoto, T.; Mandai, H.; Nagaki, A.
Beilstein J. Org. Chem. 2022, 18, 660–668.
DOI: 10.3762/bjoc.18.67
159. Glycosyl Dioxalenium Ions as Reactive Intermediates of Automated Electrochemical Assembly
Shibuya, A.; Kato, M.; Saito, A.; Manmode, S.; Nishikori, N.; Itoh, T.; Nagaki, A.; Nokami, T.
Eur. J. Org. Chem. 2022, 2022, e202200135.
DOI: 10.1002/ejoc.202200135
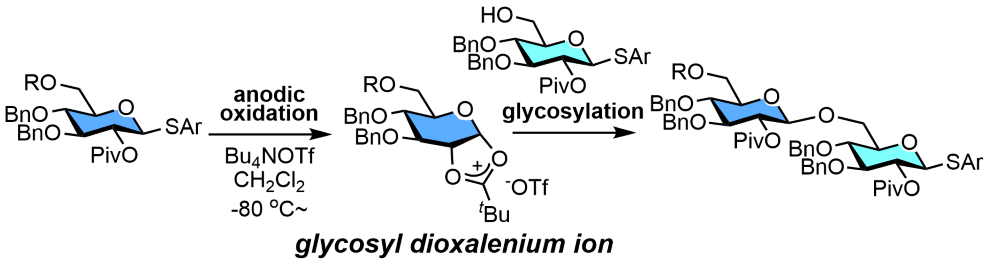
158. Flash Synthesis and Continuous Production of C-Arylglycosides in a Flow Electrochemical Reactor
Takumi, M.; Nagaki, A.
Front. Chem. Eng. 2022, 4, 862766.
DOI: 10.3389/fceng.2022.862766
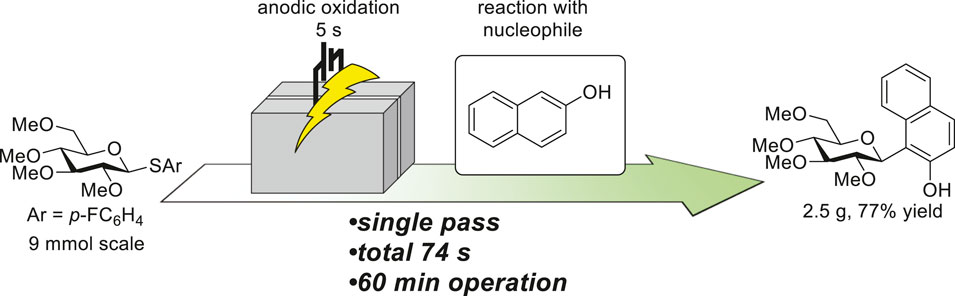
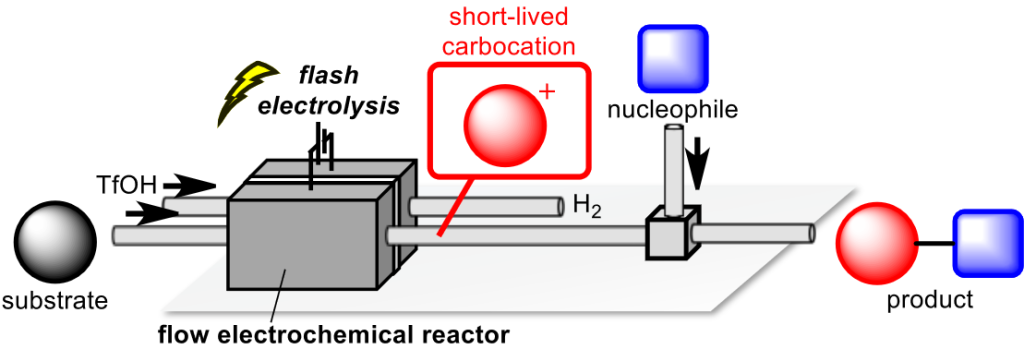
157. Flash Electrochemical Approach to Carbocations
Takumi, M.; Sakaue, H.; Nagaki, A.
Angew. Chem. Int. Ed. 2022, 61, e202116177.
DOI: 10.1002/anie.202116177
京都大学プレスリリース:「高速なフロー電気分解を駆使してわずか20秒での医薬品合成に成功」
Chem-Station:「カチオン中間体の反応に新展開をもたらす新規フロー反応装置の開発」
JST NEWS:「高速の電気分解が可能な装置を開発 医薬品前駆体を合成、わずか19秒で」
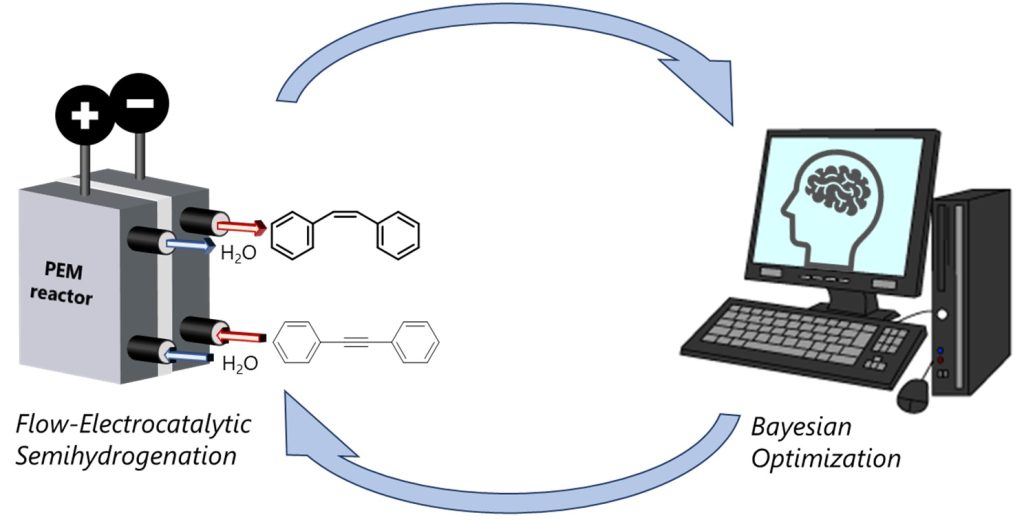
156. Investigation of Parameter Control for Electrocatalytic Semihydrogenation in a Proton-Exchange Membrane Reactor Utilizing Bayesian Optimization
Ashikari, Y.; Tamaki, T.; Takahashi, Y.; Yao, Y.; Atobe, M.; Nagaki, A.
Front. Chem. Eng. 2022, 3, 819752.
DOI: 10.3389/fceng.2021.819752
155. フローマイクロケミストリーに基づく反応集積化
永木愛一郎、芦刈洋祐
THE CHEMICAL TIMES 2022, 263, 3–6.
Link (pdf)

154. Flow grams-per-hour production enabled by hierarchical bimodal porous silica gel supported palladium column reactor having low pressure drop
Ashikari, Y.; Maekawa, K.; Takumi, M.; Tomiyasu, N.; Fujita, C.; Matsuyama, K.; Miyamoto, R.; Bai, H.; Nagaki, A.
Catal. Today 2022, 388–389, 231–236.
DOI: 10.1016/j.cattod.2020.07.014
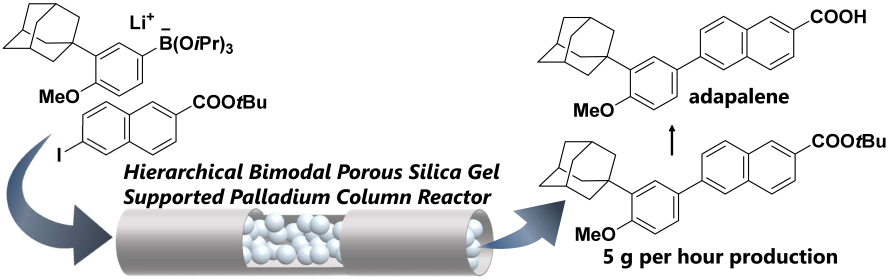
2021
153. Stille, Heck, and Sonogashira coupling and hydrogenation catalyzed by porous-silica-gel-supported palladium in batch and flow
Ashikari, Y.; Maekawa, K.; Ishibashi, M.; Fujita, C.; Shiosaki, K.; Bai, H.; Matsuyama, K.; Nagaki, A.
Green Process. Synth. 2021, 10, 722–728.
DOI: 10.1515/gps-2021-0069
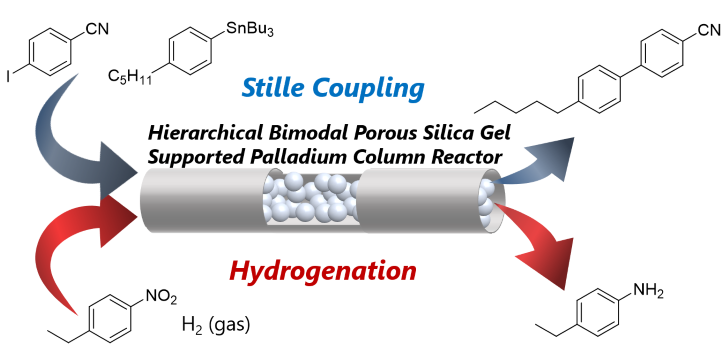
152. フロー高速合成とAI活用の将来展望について
永木愛一郎、芦刈洋祐、宅見正弘
化学工学 2021, 85, 611-614.
Link

151. Switchable Chemoselectivity of Reactive Intermediates Formation and Their Direct Use in a Flow Microreactor
Ashikari, Y.; Tamaki, T.; Kawaguchi, T.; Furusawa, M.; Yonekura, Y.; Ishikawa, S.; Takahashi, Y.; Aizawa, Y.; Nagaki, A.
Chem. Eur. J. 2021, 27, 16107–16111.
DOI: 10.1002/chem.202103183
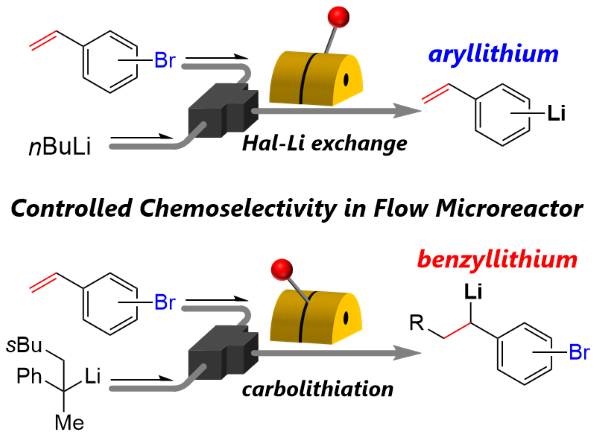
150. Flash Production of Organophosphorus Compounds in Flow
Tamaki, T.; Nagaki, A.
Tetrahedron Lett. 2021, 81, 153364.
DOI: 10.1016/j.tetlet.2021.153364
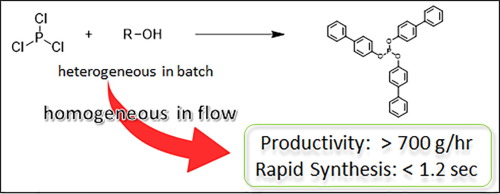
149. Principles of controlling reactions in flow chemistry
Nagaki, A.; Yoshida, J.
Vol. 1 Flow Chemistry-Fundamentals, 2nd ed. (Eds. Darvas, F.; Dormán, G.; Hessel, V.; Ley, S. V.)
Walter de Gruyter & Co., 2021年10月25日発刊, ISBN: 978-3-11-073679-3.
Link

148. 高速合成化学(第I編 第1章)
宅見正浩、永木愛一郎
147. 高分子合成反応(第I編 第6章)
芦刈洋祐、永木愛一郎
フローマイクロ合成の最新動向(監修:深瀬浩一、永木愛一郎)
シーエムシー出版、2021年8月31日発刊、ISBN:978-4-7813-1615-4.
Link

146. Multiple Organolithium Reactions based on Space Integration. (chapter 17)
Nagaki, A.
Middle Molecular Strategy: Flow Synthesis to Functional Molecules pp. 309-320.
Springer、2021年8月16日発刊、ISBN:978-981-16-2457-5
Link

145. 高速合成化学における反応選択性の制御
玉木 孝、永木 愛一郎
有機合成化学協会誌 2021, 79, 483-491.
DOI: 10.5059/yukigoseikyokaishi.79.483

144. Multiple Organolithium Reactions for Drug Discovery Using Flash Chemistry
Ashikari, Y.; Tamaki, T.; Takumi, M.; Nagaki, A.
Topics in Medicinal Chemistry 2021, Springer
DOI: 10.1007/7355_2021_113

143. Homogeneous Catalyzed Aryl–Aryl Cross-couplings in Flow
Ashikari, Y.; Nagaki, A.
Synthesis 2021, 53, 1879–1888.
DOI: 10.1055/a-1360-7798
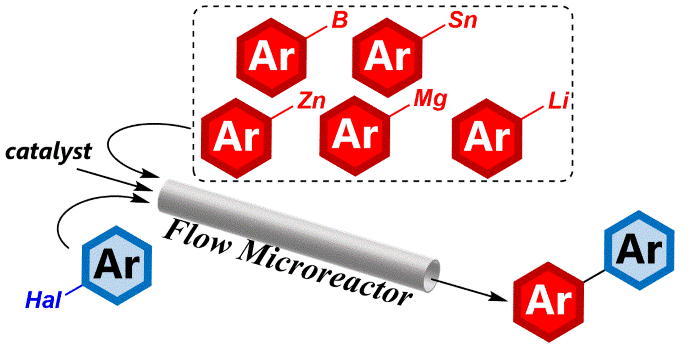
142. Flash Chemistry Makes Impossible Organolithium Chemistry Possible
Nagaki, A.; Ashikari, Y.; Takumi, M.; Tamaki, T.
Chem. Lett. 2021, 50, 485–492.
DOI: 10.1246/cl.200837
Vol. 50 Commemorative Highlight Review: Molecular Chemistry

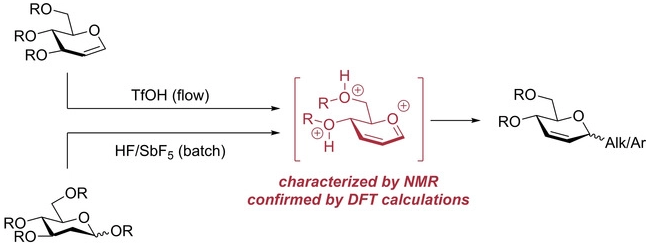
141. Insight into the Ferrier rearrangement by combining flash chemistry and superacids
Bhuma, N.; Lebedel, L.; Yamashita, H.; Shimizu, Y.; Abada, Z.; Ardá, A.; Désiré, J.; Michelet, B.; Martin-Mingot, A.; Abou-Hassan, A.; Takumi, M.; Marrot, J.; Jiménez-Barbero, J.; Nagaki, A.; Blériot, Y.; Thibaudeau, S.
Angew. Chem. Int. Ed. 2021, 60, 2036–2041.
DOI: 10.1002/anie.202010175
140. マイクロチャネル:マイクロ混合・反応(第2編第4章第3節)
芦刈洋祐、永木愛一郎
マイクロ・ナノ熱工学の進展
株式会社エヌ・ティー・エス、2021年5月19日発刊
ISBN:978-4-86043-722-0
Link

2020
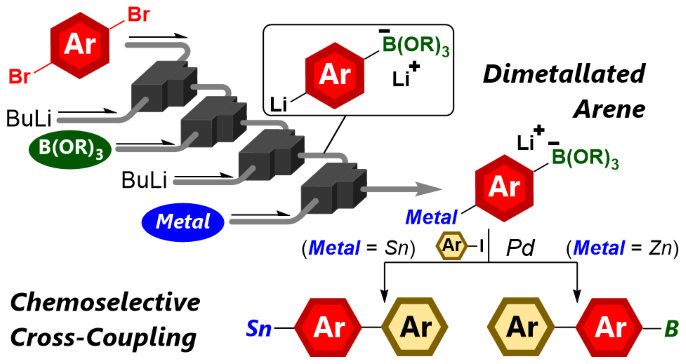
139. A Synthetic Approach to Dimetallated Arenes Using Flow Microreactors and the Switchable Application to Chemoselective Cross-Coupling Reactions
Ashikari, Y.; Kawaguchi, T.; Mandai, K.; Aizawa, Y.; Nagaki, A.
J. Am. Chem. Soc. 2020, 142, 17039–17047.
DOI: 10.1021/jacs.0c06370
Selected as a Cover Picture
Highlighted in 現代化学 2020, 597, 11.
「マイクロフロー合成を利用してクロスカップリング反応」

138. Accelerating Heat-Initiated Radical Reactions of Organic Halides with Tin Hydride Using Flow Microreactor Technologies
Jiang, Y.; Ashikari, Y.; Guan, K.; Nagaki, A.
Synlett 2020, 31, 1937–1941.
DOI: 10.1055/s-0040-1707307
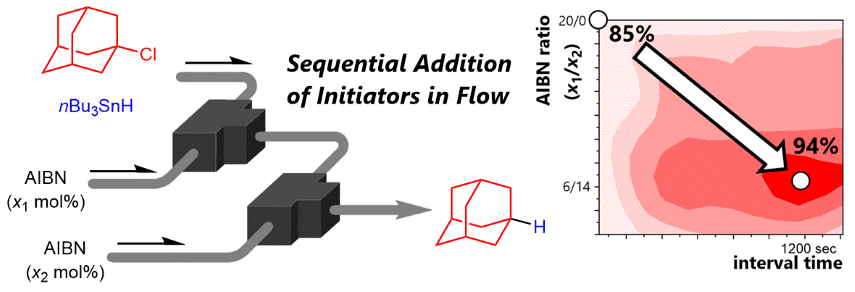
137. Trapping of Transient Thienyllithiums Generated by Deprotonation of 2,3- and 2,5-Dibromothiophenes in a Flow Microreactor
Okano, K.; Yamane, Y.; Nagaki, A.; Mori, A.
Synlett 2020, 31, 1913–1918.
DOI: 10.1055/s-0040-1706479
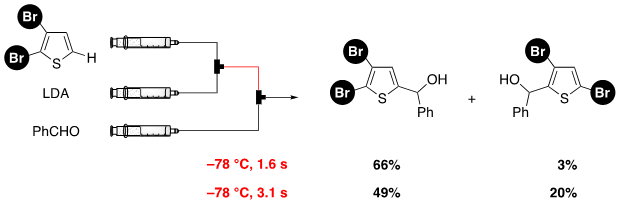
136. Pd catalysts supported on dual-pore monolithic silica beads for chemoselective hydrogenation under batch and flow reaction conditions
Yamada, T.; Ogawa, A.; Masuda, H.; Teranishi, W.; Fujii, A.; Park, K.; Ashikari, Y.; Tomiyasu, N.; Ichikawa, T.; Miyamoto, R.; Bai, H.; Matsuyama, K.; Nagaki, A.; Sajiki, H.
Catal. Sci. Technol. 2020, 10, 6359–6367.
DOI: 10.1039/D0CY01442G
135. マイクロリアクター、フロー合成分野の動向と展望(第1章)
永木愛一郎
134. フローリアクターを用いた合成反応、プロセス設計と応用例(第6章1節)
萬代恭子、永木愛一郎
133. フローマイクロリアクターの高速混合を利用した高選択的化学反応(第6章2節)
芦刈洋祐、永木愛一郎
132. フローリアクターを用いた不安定中間体を経由する反応集積化(第6章3節)
芦刈洋祐、永木愛一郎
131. フローリアクターを用いた有機電解合成(第6章4節)
宅見正浩、永木愛一郎
130. フローリアクターを用いた鈴木-宮浦カップリング反応(第6章6節)
前川圭、芦刈洋祐、永木愛一郎
129. フロー精密アニオン重合技術と実用化に向けた課題(第6章12節)
高橋祐輔、永木愛一郎
フロー合成、連続生産のプロセス設計、条件設定と応用事例
技術情報協会、2020年12月25日発刊、ISBN:978-4-86104-820-3
(オンデマンド版ISBN:978-4-86104-946-0)
Link

128. 時間を空間で制御する高分子合成化学
永木愛一郎、高橋祐輔
高分子 2020, 69, 355-358.
Link

127. Fluoro-Substituted Methyllithium Chemistry: External Quenching Method Using Flow Microreactors
Colella, M.; Tota, A.; Takahashi, Y.; Higuma, R.; Ishikawa, S.; Degennaro, L.; Luisi, R.; Nagaki, A.
Angew. Chem. Int. Ed. 2020, 59, 10924–10928.
DOI: 10.1002/anie.202003831
Highlighted in Synfacts
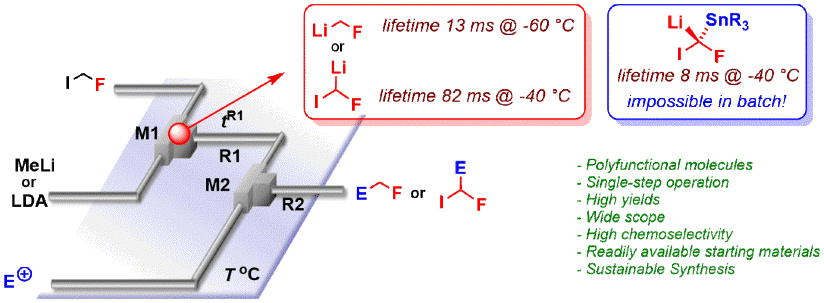
126. Bromine-lithium exchange on a gem-dibromoalkene. Part 2: comparative performance of flow micromixers
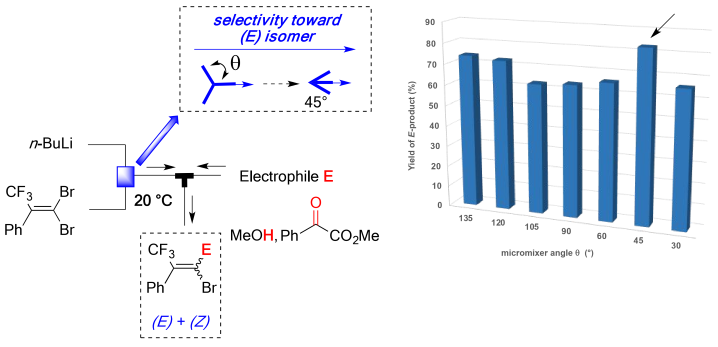
Perez, K.; Picard, B.; Vuluga, D.; Burel, F.; Hreiz, R.; Falk, L.; Commenge, J.-M.; Nagaki, A.; Yoshida, J.; Chataigner, I.; Maddaluno, J.; Legros, J.
Org. Process Res. Dev. 2020, 24, 787–791.
DOI: 10.1021/acs.oprd.0c00203
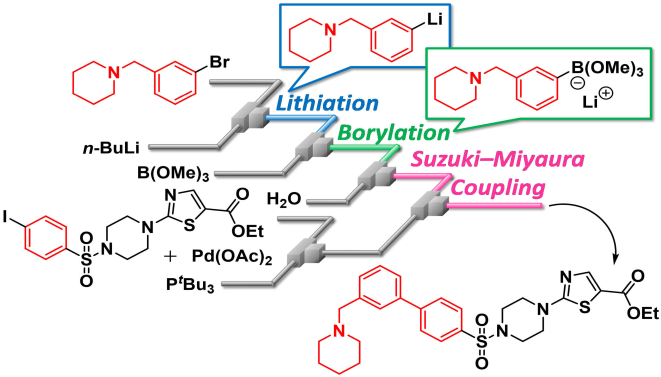
125. Synthesis of Biaryls Having a Piperidylmethyl Group Based on Space Integration of Lithiation, Borylation and Suzuki-Miyaura Coupling
Takahashi, Y.; Ashikari, Y.; Takumi, M.; Shimizu, Y.; Jiang, Y.; Higuma, R.; Ishikawa, S.; Sakaue, H.; Shite, I.; Maekawa, K.; Aizawa, Y.; Yamashita, H.; Yonekura, Y.; Colella, M.; Luisi, R.; Takegawa, T.; Fujita, C.; Nagaki, A.
Eur. J. Org. Chem. 2020, 618–622.
DOI: 10.1002/ejoc.201901729
124. Continuous Production Using a T-shaped Micro/milli-reactor for RUCY-catalyzed Asymmetric Hydrogenation of Acetophenone
Yamamoto, T.; Tonomura, O.; Nagaki, A.
J. Chem. Eng. Jpn. 2020, 53, 73–77.
DOI: 10.1252/jcej.19we083

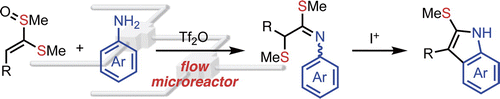
123. Tf2O-mediated Reaction of Alkenyl Sulfoxides with Unprotected Anilines in Flow Microreactors
Baralle, A.; Inukai, T.; Yanagi, T.; Nogi, K.; Osuka, A.; Nagaki, A.; Yoshida, J.; Yorimitsu, H.
Chem. Lett. 2020, 49, 160–163.
DOI: 10.1246/cl.190831
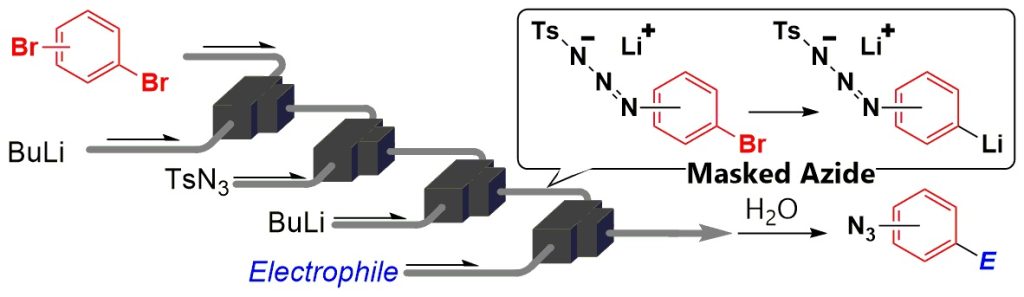
122. A Novel Approach to Functionalization of Aryl Azides throuth the Generation and Reactions of Organolithiums Bearing Masked Azides Using Flow Microreactors
Ichinari, D.; Ashikari, Y.; Mandai, K.; Aizawa, Y.; Yoshida, J.; Nagaki, A.
Angew. Chem. Int. Ed. 2020, 59, 1567–1571.
DOI: 10.1002/anie.201912419
Highlighted in Synfacts
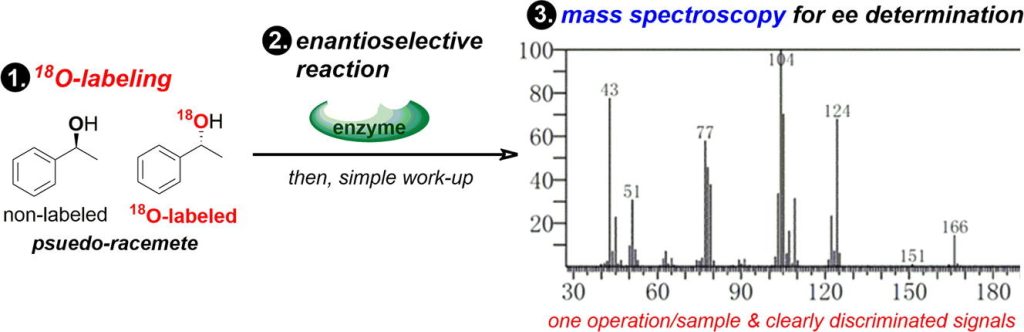
121. 18O-Labeled chiral compounds enable the facile determination of enantioselectivity by mass spectroscopy
Mandai, K.; Tsuchihashi, Y.; Ashikari, Y.; Yoshida, J.; Nagaki, A.
Tetrahedron Lett. 2020, 61, 151367.
DOI: 10.1016/j.tetlet.2019.151367
120. Flow Technology for Genesis and Use of (Highly) Reactive Organometallic Reagents
Colella, M.; Nagaki, A.; Luisi, R.
Chem. Eur. J. 2020, 26, 19–32.
DOI: 10.1002/chem.201903353
119. フロー自動合成とAI(人工知能)を活用した研究・開発 ~現状の課題と将来展望~(第1部第1章)
永木愛一郎、清水悠、宅見正浩
118. 有機合成への応用技術と実用化事例(第2部第1章)
芦刈洋祐、永木愛一郎
マイクロリアクター/フロー合成による反応条件を最適化した導入と目的に応じた実生産への適用~事例をふまえた現状と課題 / 不具合を避けるための設備設計~
サイエンス&テクノロジー社、2020年4月28日発刊、ISBN:978-4-86428-211-6
Link

2019
117. Oxo-Thiolation of Cationically Polymerizable Alkenes Using Flow Microreactors
Ashikari, Y.; Saito, K.; Nokami, T.; Yoshida, J.; Nagaki, A.
Chem. Eur. J. 2019, 25, 15239–15243.
DOI: 10.1002/chem.201903426
Selected as a cover picture
Link
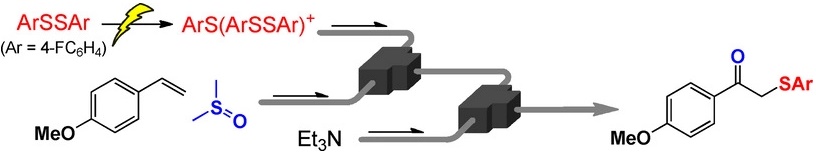

116. Generation and Reaction of Functional Alkyllithiums Using Microreactors and Their Application to Heterotelechelic Polymer Synthesis
Nagaki, A.; Yamashita, H.; Hirose, K.; Tsuchihashi, Y.; Takumi, M.; Yoshida, J.
Chem. Eur. J. 2019, 25, 13719–13727.
DOI: 10.1002/chem.201902867
Selected as a cover picture
Cover Profile
Highlighted in Synfacts
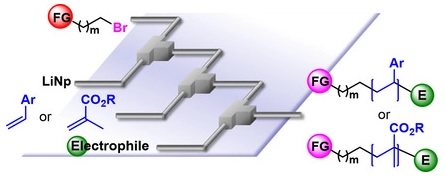

115. Recent Topics of Functionalized Organolithiums using Flow Microreactor Chemistry
Nagaki, A.
Tetrahedron Lett. 2019, 60, 150923.
DOI: 10.1016/j.tetlet.2019.07.014
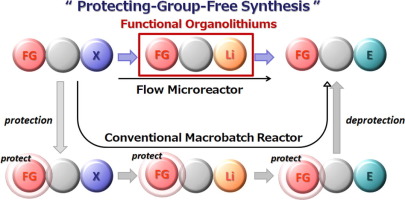
114. Practical Continuous Flow Controlled/Living Anionic Polymerization
Nakahara, Y.; Furusawa, M.; Endo, Y.; Shimazaki, T.; Takahashi, Y.; Jiang, Y.; Nagaki, A.
Chem. Eng. Technol. 2019, 42, 2154–2163.
DOI: 10.1002/ceat.201900160
113. Anionic Polymerizations using Flow Microreactors
Takahashi, Y.; Nagaki, A.
Molecules 2019, 24, 1532.
DOI: 10.3390/molecules24081532
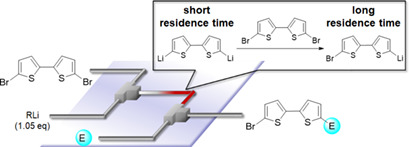
112. Monolithiation of 5,5’-Dibromo-2,2’-bithiophene Using Flow Microreactors. Mechanistic Implications and Synthetic Applications
Nagaki, A.; Jiang, Y.; Yamashita, H.; Takabayashi, N.; Yoshida, J.
Chem. Eng. Technol. 2019, 42, 2113–2118.
DOI: 10.1002/ceat.201900057
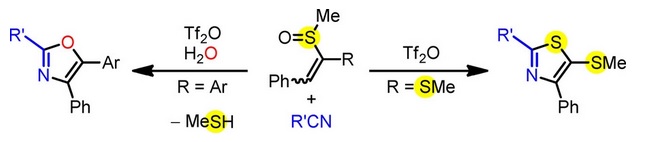
111. Annulative Synthesis of Thiazoles and Oxazoles from Alkenyl Sulfoxides and Nitriles via Additive Pummerer Reaction
Hori, M.; Nogi, K.; Nagaki, A.; Yorimitsu, H.
Asian J. Org. Chem. 2019, 8, 1084–1087.
DOI: 10.1002/ajoc.201900169
110. Blockage Detection and Diagnosis of Externally Parallelized Monolithic Microreactors
Tonomura, O.; Taniguchi, S.; Nishi, K.; Nagaki, A.; Yoshida, J.; Hirose, K.; Ishizuka, N.; Hasebe, S.
Catalysts 2019, 9, 308.
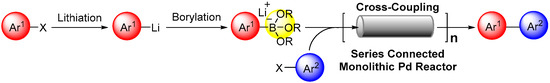
DOI: 10.3390/catal9040308
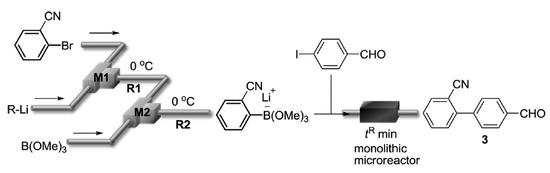
109. Suzuki–Miyaura Coupling Using Monolithic Pd Reactors and Scaling-up by Series Connection of the Reactors
Nagaki, A.; Hirose, K.; Mitamura, K.; Matsukawa, K.; Ishizuka, N.; Yamamoto, T.; Takumi, M.; Takahashi, Y.; Yoshida, J.
Catalysts 2019, 9, 300.
DOI: 10.3390/catal9030300
108. Alkyllithiums Bearing Electrophilic Functional Groups: A Flash Chemistry Approach.
Nagaki, A.; Yamashita, H.; Hirose, K.; Tsuchihashi, Y.; Yoshida, J.
Angew. Chem. Int. Ed. 2019, 58, 4027–4030.
DOI: 10.1002/anie.201814088
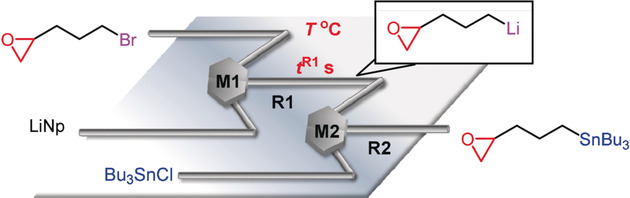
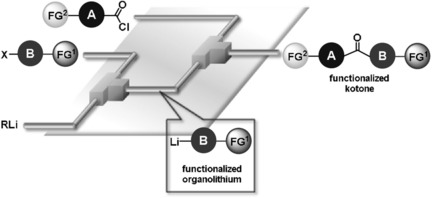
107. Synthesis of Functionalized Ketones from Acid Chlorides and Organolithiums by Extremely Fast Micromixing.
Nagaki, A.; Sasatsuki, K.; Ishiuchi, S.; Miuchi, N.; Takumi, M.; Yoshida, J.
Chem. Eur. J. 2019, 25, 4946–4950.
DOI: 10.1002/chem.201900743
Highlighted in Synfacts
106. マイクロリアクターの研究開発状況とその展望
宅見正浩、永木愛一郎
「化学装置」工業通信, 2019, 61, 17–22.

105. Modeling and Design of Flow Microreactor-based Process for Synthesizing Ionic Liquids.
Nakahara, Y.; Metten, B.; Tonomura, O.; Nagaki, A.; Hasebe, S.; Yoshida, J.
Org. Process Res. Dev. 2019, 23, 641–647.
DOI: 10.1021/acs.oprd.8b00436
104. Molecular Weight Distribution of Polymers Produced by Anionic Polymerization Enables Mixability Evaluation.
Endo, Y.; Furusawa, M.; Shimazaki, T.; Takahashi, Y.; Nakahara, Y.; Nagaki, A.
Org. Process Res. Dev. 2019, 23, 635–640.
DOI: 10.1021/acs.oprd.8b00403
103. フローマイクロリアクターを用いた有機合成反応とその選択性制御(第8章1節)
高橋裕輔、永木愛一郎
102. フローマイクロリアクターを用いた高分子合成反応とその連続運転(第8章4節)
永木愛一郎、中原祐一、遠藤裕太、高橋 裕輔
化学プロセスのスケールアップ、連続化 ―データ取得/装置・プロセス設計/生産性向上/トラブル対策―
技術情報協会、2019年3月31日発刊
ISBN: 978-4-86104-739-8. Link

2018
101. 高速マイクロ混合による反応選択性の高次制御
永木愛一郎、宅見正浩
月刊ファインケミカル2018年12月号「フローマイクロ合成の最新動向I」
シーエムシー出版、2018年12月15日発刊
ISBN: 0913-6150. LINK

100. Transmission of Point Chirality to Axial Chirality for Strong Circular Dichroism in Triarylmethylium-o,o-dimers
Ishigaki, Y.; Iwai, T.; Hayashi, Y.; Nagaki, A.; Katoono, R.; Fujiwara, K.; Yoshida, J.; Suzuki, T.
Synlett 2018, 29, 2147–2154.
DOI: 10.1055/s-0037-1610190
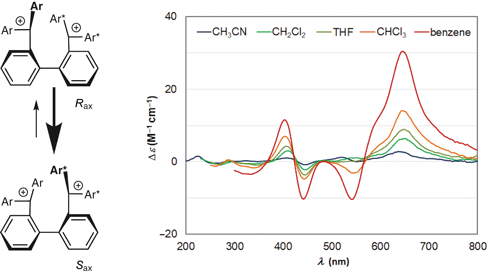
99. Efficient Preparation of A Cyclic α-Alkylidene β-Oxo Imides Using a Microflow System
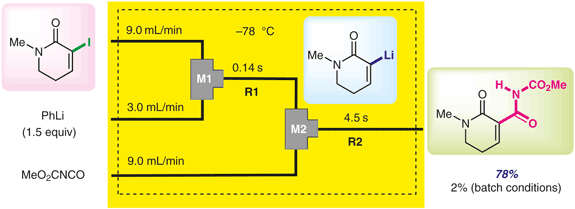
Komuro, K.; Nagaki, A.; Shimoda, H.; Uwamori, M.; Yoshida, J.; Nakada, M.
Synlett 2018, 29, 1989–1994.
DOI: 10.1055/s-0037-1610228
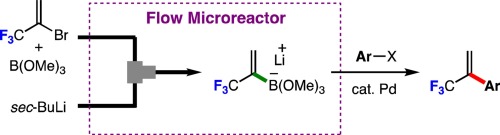
98. Flash Generation and Borylation of 1-(Trifluoromethyl)Vinyllithium toward Synthesis of α-(Trifluoromethyl)Styrenes
Fujita, T.; Konno, N.; Watabe, Y.; Ichitsuka, T.; Nagaki, A.; Yoshida, J.; Ichikawa, J.
J. Fluorine Chem. 2018, 207, 72–76.
DOI: 10.1016/j.jfluchem.2018.01.004
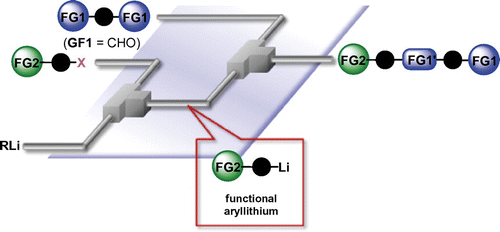
97. Selective Mono Addition of Aryllithiums to Dialdehydes by Micromixing
Nagaki, A.; Yamashita, H.; Takahashi, Y.; Ishiuchi, S.; Imai, K.; Yoshida, J.
Chem. Lett. 2018, 47, 71–73.
DOI: 10.1246/cl.170899
2017
96. “Impossible” Chemistries Based on Flow and Micro
Yoshida, J.; Kim, H.; Nagaki, A.
J. Flow Chem. 2017, 7, 60–64.
DOI: 10.1556/1846.2017.00017
95. Micromixing Enables Chemoselective Reactions of Difunctional Electrophiles with Functional Aryllithiums
Nagaki, A.; Ishiuchi, S.; Imai, K.; Sasatsuki, K.; Nakahara, Y.; Yoshida, J.
React. Chem. Eng. 2017, 2, 862–870.
DOI: 10.1039/C7RE00142H
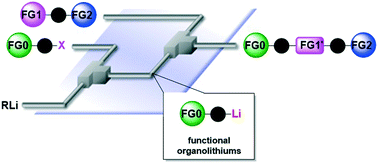
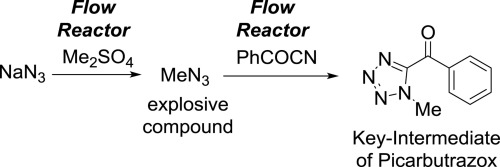
94. Generation of Hazardous Methyl Azide and Its Application to Synthesis of a Key-Intermediate of Picarbutrazox, a New Potent Pesticide in Flow
Ichinari, D.; Nagaki, A.; Yoshida, J.
Bio. Med. Chem. 2017, 25, 6224–6228.
DOI: 10.1016/j.bmc.2017.07.005
93. フローマイクロリアクターの化学業界の動向(第III編第2章)
金熙珍、永木愛一郎、吉田潤一
フローマイクロ合成の実用化への展望(監修:吉田潤一)
シーエムシー出版、2017年1月11日発刊
ISBN: 978-4-7813-1232-3. Link

2016
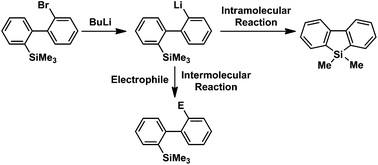
92. Switching Between Intermolecular and Intramolecular Reactions Using Flow Microreactors. Lithiation of 2-Bromo-2′-Silylbiphenyls
Nagaki, A.; Kim, S.; Miuchi, N.; Yamashita, H.; Hirose, K.; Yoshida, J.
Org. Chem. Front. 2016, 3, 1250–1253.
DOI: 10.1039/C6QO00257A
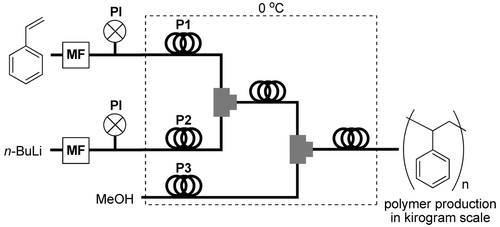
91. Feasibility Study on Continuous Flow Controlled/Living Anionic Polymerization Processes.
Nagaki, A.; Nakahara, Y.; Furusawa, M.; Sawaki, T.; Yamamoto, T.; Toukairin, H.; Tadokoro, S.; Shimazaki, T.; Ito, T.; Otake, M.; Arai, H.; Higashida, N.; Takahashi, Y.; Moriwaki, Y.; Tsuchihashi, Y.; Hirose, K.; Yoshida, J.
Org. Process Res. Dev. 2016, 20, 1377–1382.
DOI: 10.1021/acs.oprd.6b00158
90. Generation and Reaction of Carbamoyl Anions in Flow: Applications in the Three-Component Synthesis of Functionalized a-Ketoamides.
Nagaki, A.; Takahashi, Y.; Yoshida, J.
Angew. Chem., Int. Ed. 2016, 55, 5327–5331
DOI: 10.1002/anie.201601386
Selected as a cover picture
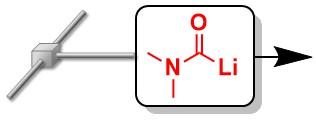

89. Integration of Borylation of Aryllithiums and Suzuki-Miyaura Coupling Using Monolithic Pd Catalyst
Nagaki, A.; Hirose, K.; Moriwaki, Y.; Mitamura, K.; Matsukawa, K.; Ishizuka, N.; Yoshida, J.
Catal. Sci. Technol. 2016, 6, 4690–4694.
DOI: 10.1039/C5CY02098K
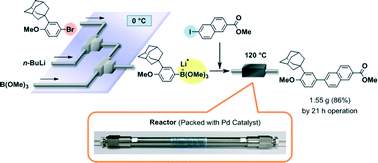
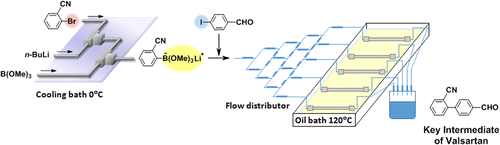
88. Design of a Numbering-up System of Monolithic Microreactors and Its Application to Synthesis of a Key Intermediate of Valsartan
Nagaki, A.; Hirose, K.; Tonomura, O.; Taga, T.; Taniguchi, S.; Hasebe, S.; Ishizuka, N.; Yoshida, J.
Org. Process Res. Dev. 2016, 20, 687–691.
DOI: 10.1021/acs.oprd.5b00414
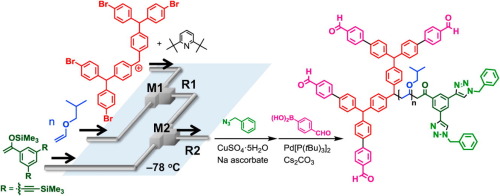
87. Flash Cationic Polymerization Followed by Bis-end-functionalization. A New Approach to Linear-Dendritic Hybrid Polymers.
Tani, Y.; Takumi, M.; Moronaga, S.; Nagaki, A.; Yoshida, J.
Eur. Poly. J. 2016, 80, 227–233.
DOI: 10.1016/j.eurpolymj.2016.02.021
86. Flow microreactor synthesis of 2,2-disubstituted oxetanes via 2-phenyloxetan-2-yl lithium
Degennaro, L.; Nagaki, A.; Moriwaki, Y.; Romanazzi, G.; DelľAnna, M. M.; Yoshida, J.; Luisi, R.
Open Chem. 2016, 14, 377–382.
DOI: 10.1515/chem-2016-0041

85. Organometallic Flow Chemistry
Nagaki, A.; Yoshida, J.
Top. Organomet. Chem. 2016, 57, 137–175.
DOI: 10.1007/3418_2015_154

84. Flow Microreactor Polymerization
Nagaki, A.
ケミカルエンジニヤリング 2016, 61(9), 683–692.

83. マイクロ流路を利用した空間的反応集積化
吉田潤一、金熙珍、永木愛一郎
化学と工業 2016, 69, 117–119. Link (pdf)

2015
82. Polymerization of Vinyl Ethers Initiated by Dendritic Cations Using Flow Microreactors
Nagaki, A.; Takumi, M.; Tani, Y.; Yoshida, J.
Tetrahedron 2015, 71, 5973–5978.
DOI: 10.1016/j.tet.2015.05.096
81. Organolithiums Bearing Aldehyde Carbonyl Groups. A Flash Chemistry Approach
Nagaki, A.; Tsuchihashi, Y.; Haraki, S.; Yoshida, J.
Org. Biomol. Chem. 2015, 13, 7140–7145.
DOI: 10.1039/C5OB00958H
80. Reactions of Difunctional Electrophiles with Functionalized Aryllithium Compounds: Remarkable Chemoselectivity by Flash Chemistry.
Nagaki, A.; Imai, K.; Ishiuchi, S.; Yoshida, J.
Angew. Chem., Int. Ed. 2015, 54, 1914–1918.
DOI: 10.1002/anie.201410717
79. Flash Chemistry Using Trichlorovinyllithium. Switching the Reaction Pathways by High-Resolution Reaction Time Control.
Nagaki, A.; Takahashi, Y.; Henseler, A.; Matsuo, C.; Yoshida, J.
Chem. Lett. 2015, 44, 214–216.
DOI: 10.1246/cl.140980
78. Reaction Integration Using Electrogenerated Cationic Intermediates.
Yoshida, J.; Shimizu, A.; Ashikari, Y.; Morofuji, T.; Hayashi, R.; Nokami, T.; Nagaki, A.
Bull. Chem. Soc. Jpn. 2015, 88, 763-775.
DOI: 10.1246/bcsj.20150100
77. Synthetic Chemistry in Flow Microreactors.
Nagaki, A.
J. Syn. Org. Chem., Jpn. 2015, 73, 423-434.
DOI: 10.5059/yukigoseikyokaishi.73.423

76. 高速マイクロ混合とフロー合成
吉田潤一、永木愛一郎
ながれ 2015, 34, 3–9.
75. フラッシュケミストリー──フラスコではできない合成化学を目指して
吉田潤一、永木愛一郎、金熙珍、市成大輔
月刊化学 2015年4月号(2015/03/18発刊)Link

2014
74. Three-Component Coupling Based on Flash Chemistry. Carbolithiation of Benzyne with Functionalized Aryllithiums Followed by Reactions with Electrophiles
Nagaki, A.; Ichinari, D.; Yoshida, J.
J. Am. Chem. Soc. 2014, 136, 12245–12248.
DOI: 10.1021/ja5071762
73. Flow Microreactor Synthesis of Fluorine-Containing Block Copolymers
Nagaki, A.; Akahori, K.; Takahashi, Y.; Yoshida, J.
J. Flow Chem. 2014, 4, 168–172.
DOI: 10.1556/JFC-D-14-00017
72. Flash Generation of α-(Trifluoromethyl)vinyllithium and Application to Continuous Flow Three-Component Synthesis of α-Trifluoromethylamides
Nagaki, A.; Tokuoka, S.; Yoshida, J.
Chem. Commun. 2014, 50, 15079–15081.
DOI: 10.1039/C4CC06709F
71. Extremely Fast Gas/Liquid Reactions in Flow Microreactors: Carboxylation of Short-Lived Organolithiums
Nagaki, A.; Takahashi, Y.; Yoshida, J.
Chem. Eur. J. 2014, 20, 7931–7934.
DOI: 10.1002/chem.201402520
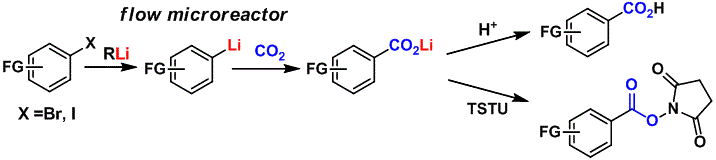
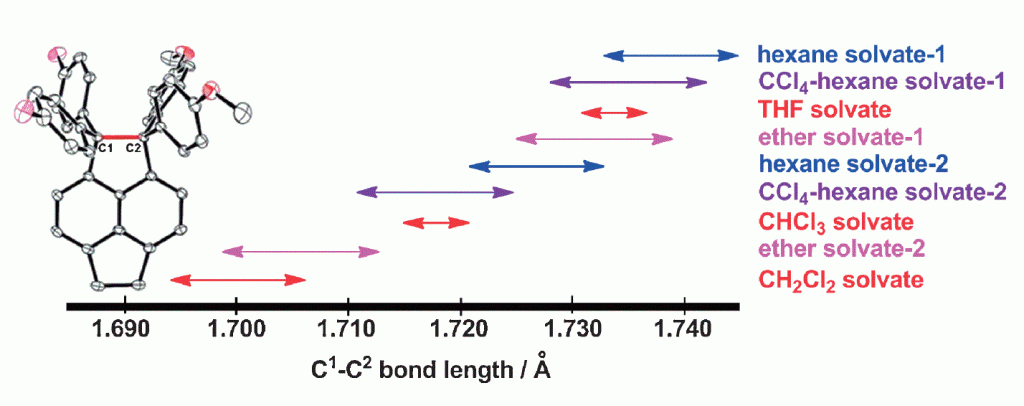
70. Expandability of Ultralong CC Bonds: Largely Different C1C2 Bond Lengths Determined by Low-temperature X-ray Structural Analyses on Pseudopolymorphs of 1,1-Bis(4-fluorophenyl)-2,2-bis(4-methoxyphenyl)pyracene
Suzuki, T.; Uchimura, Y.; Nagasawa, F.; Takeda, T.; Kawai, H.; Katoono, R.; Fujiwara, K.; Murakoshi, K.; Fukushima, T.; Nagaki, A.;Yoshida, J.
Chem. Lett. 2014, 43, 86–88.
DOI: 10.1246/cl.130872
69. 有機金属反応(第11章)
永木愛一郎
68. 重合反応(第18章)
永木愛一郎
フロー・マイクロ合成 基礎から実際の合成・製造まで(吉田潤一 編)
化学同人、2014年7月24日発刊、ISBN: 978-4759814170

67. Microreactor Technology in Lithium Chemistry (Chapter 17)
Nagaki, A.; Yoshida, J.
Lithium Compounds in Organic Synthesis – From Fundamentals to Applications (Edited by Renzo Luisi and Vito Capriati)
Wiley-VCH, published in March 19 2014, ISBN: 978-3527333431

2013
66. Reactions of Organolithiums with Dialkyl Oxalates. A Flow Microreactor Approach to Synthesis of Functionalized a-Keto Esters
Nagaki, A.; Ichinari, D.; Yoshida, J.
Chem. Commun. 2013, 49, 3242–3244.
DOI: 10.1039/c3cc40392k
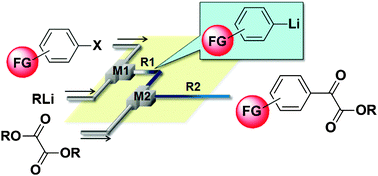
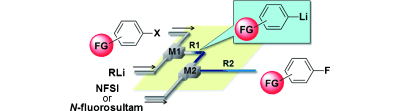
65. Synthesis of Functionalized Aryl Fluorides Using Organolithium Reagents in Flow Microreactors
Nagaki, A.; Uesugi, Y.; Kim, H.; Yoshida, J.
Chem. Asian J. 2013, 8, 705–708.
DOI: 10.1002/asia.201201191
64. Generation and Reactions of Pyridyllithiums via Br/Li Exchange Reactions Using Continuous Flow Microreactor Systems
Nagaki, A.; Yamada, D.; Yamada, S.; Doi, M.; Ichinari, D.; Tomida, Y.; Takabayashi, N.; Yoshida, J.
Aust. J. Chem. 2013, 66, 199–207.
DOI: 10.1071/CH12440
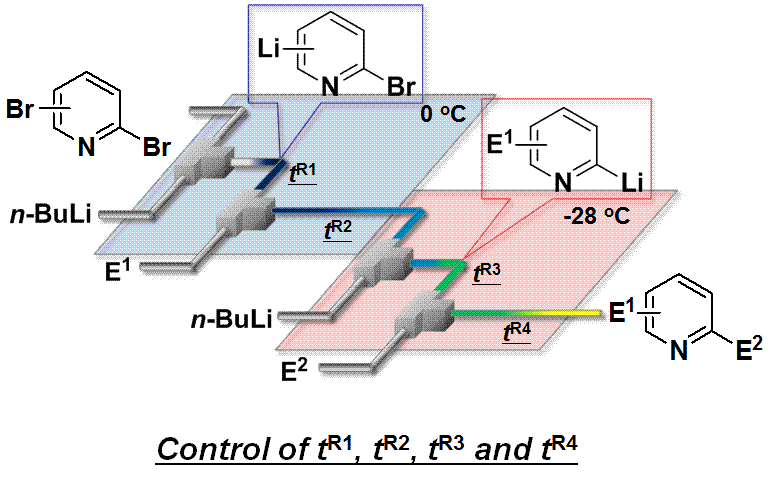
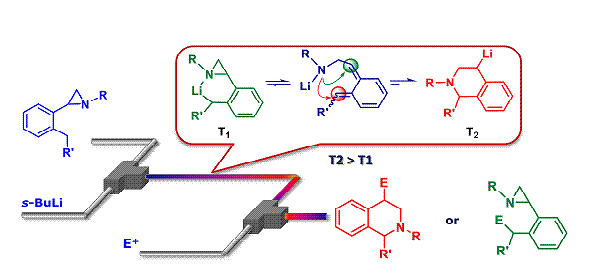
63. Synthesis of 1,2,3,4-Tetrahydroisoquinolines by Microreactor-Mediated Thermal Isomerization of Laterally Lithiated Arylaziridines
Giovine, A.; Musio, B.; Degennaro, L.; Falcicchio, A.; Nagaki, A.; Yoshida, J.; Luisi, R.
Chem. Eur. J. 2013, 19, 1872–1876.
DOI: 10.1002/chem.201203533
62. Controlled Polymerization in Flow Microreactor Systems.
Nagaki, A.; Yoshida, J.
Controlled Polymerization and Polymeric Structures, Vol. 259, Springer-Verlag, 2013, pp1–50.
DOI: 10.1007/12_2012_179

61. マイクロリアクターによる有機リチウム化学の新展開
永木 愛一郎
有機合成化学協会誌 2013, 71, 1002–1019.
DOI: 10.5059/yukigoseikyokaishi.71.1002

60. マイクロリアクターによる有機リチウム化学の新展開
永木愛一郎
化学と工業 2013, 66 (11), 924–925. Link

59. Continuous Flow Synthesis
Yoshida, J.; Nagaki, A.; Yamada, D.
Drug Discovery Today, 2013, 10, e53–e59.
DOI: 10.1016/j.ddtec.2012.10.013
58. Flow Microreactor Synthesis in Organofluorine Chemistry
Amii, H.; Nagaki, A.; Yoshida, J.
Beilstein J. Org. Chem. 2013, 9, 2793–2802.
DOI: 10.3762/bjoc.9.314
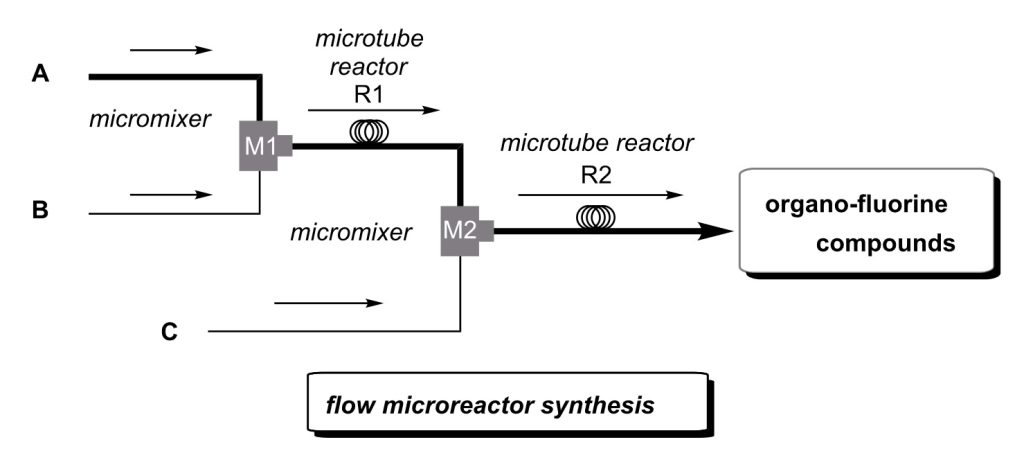
57. Flash Chemistry: Flow Chemistry That Cannot Be Done in Batch
Yoshida, J.; Takahashi, Y.; Nagaki, A.
Chem. Commun. 2013, 49, 9896–9904.
DOI: 10.1039/C3CC44709J
56. マイクロリアクターによる有機リチウム化学への新展開
永木愛一郎
化学とマイクロ・ナノシステム学会誌 2013, 12 (2), 30.
55. Flash Chemistry: New Synthetic Chemistry Using Flow Microreactors
Yoshida, J.; Takahashi, Y.; Nagaki, A.
Kagaku Kogaku 2013, 77, 785–787.
54. Electrochemical Reactions in Microreactors (Chapter 9)
Yoshida, J.; Nagaki, A.
Microreactors in Preparative Chemistry (edited by Wladimir Reschetilowski)
Wiley-VCH, published July 19 2013, ISBN: 9783527332823. Link

2012
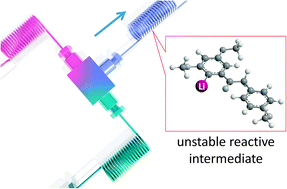
53. Lithiation of 1,2-Dichloroethene in Flow Microreactors. Versatile Synthesis of Alkenes and Alkynes by Precise Residence-Time Control.
Nagaki, A.; Matsuo, C.; Kim, S.; Saito, K.; Miyazaki, A.; Yoshida, J.
Angew. Chem. Int. Ed. 2012, 51, 3245–3248.
DOI: 10.1002/anie.201108932
52. Flow Synthesis of Arylboronic Esters Bearing Electrophilic Functional Groups and Space Integration with Suzuki–Miyaura Coupling without Intentionally Added Base.
Nagaki, A.; Moriwaki, Y.; Yoshida, J.
Chem. Commun. 2012, 48, 11211–11213.
DOI: 10.1039/C2CC36197C
51. Flash Generation of a Highly Reactive Pd Catalyst for Suzuki–Miyaura Coupling by Using a Flow Microreactor.
Nagaki, A.; Takabayashi, N.; Moriwaki, Y.; Yoshida, J.
Chem. Eur. J. 2012, 18, 11871–11875.
DOI: 10.1002/chem.201201579
50. Practical Synthesis of Photochromic Diarylethenes in Integrated Flow Microreactor Systems.
Asai, T.; Takata, A.; Nagaki, A.; Yoshida, J.
ChemSusChem 2012, 5, 339–350.
DOI: 10.1002/cssc.201100376
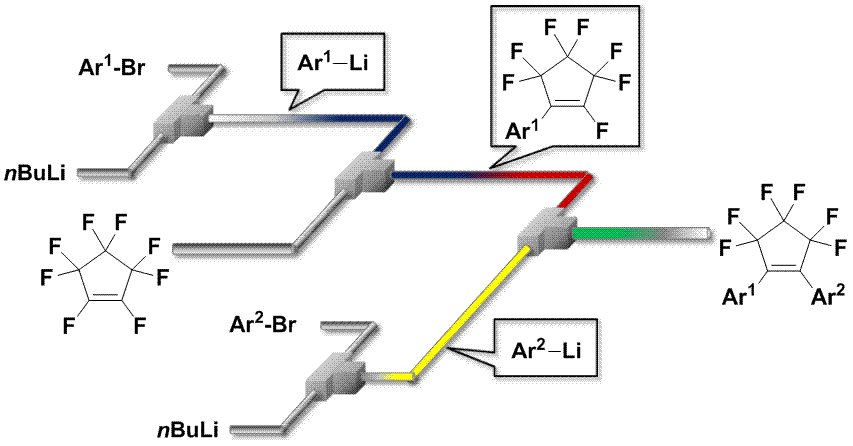

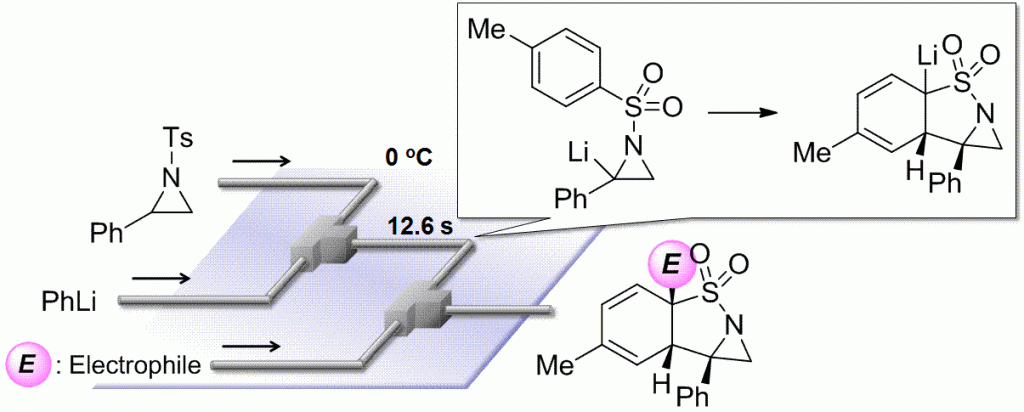
49. Flow Microreactor Synthesis of Tricyclic Sulfonamides via N-tosylaziridinyllithiums.
Takizawa, E.; Nagaki, A.; Yoshida, J.
Tetrahedron Lett. 2012, 53, 1397–1400.
DOI: 10.1016/j.tetlet.2012.01.019
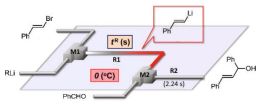
48. Cross-coupling of Aryllithiums with Aryl and Vinyl Halides in Flow Microreactors.
Nagaki, A.; Moriwaki, Y.; Haraki, S.; Kenmoku, A.; Hayashi, A.; Yoshida, J.
Chem. Asian J. 2012, 7, 1061–1068.
DOI: 10.1002/asia.201101019
47. Generation and Reactions of Vinyllithiums Using Flow Microreactor Systems.
Nagaki, A.; Takahashi, Y.; Yamada, S.; Matsuo, C.; Haraki, S.; Moriwaki, Y.; Kim, S.; Yoshida, J.
J. Flow Chem. 2012, 2, 70–72.
DOI: 10.1556/JFC-D-12-00004
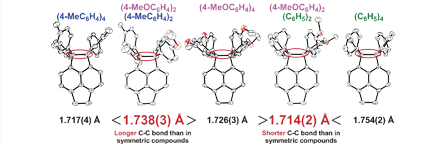
46. Non-additive Substituent Effects on Expanding Prestrained C–C Bond in Crystal: X-ray Analyses on Unsymmetrically Substituted Tetraarylpyracenes Prepared by a Flow Microreactor Method
Suzuki, T.; Uchimura, Y.; Ishigaki, Y.; Takeda, T.; Katoono, R.; Kawai, H.; Fujiwara, K.; Nagaki, A.; Yoshida, J.
Chem. Lett. 2012, 41, 541–543.
DOI: 10.1246/cl.2012.541
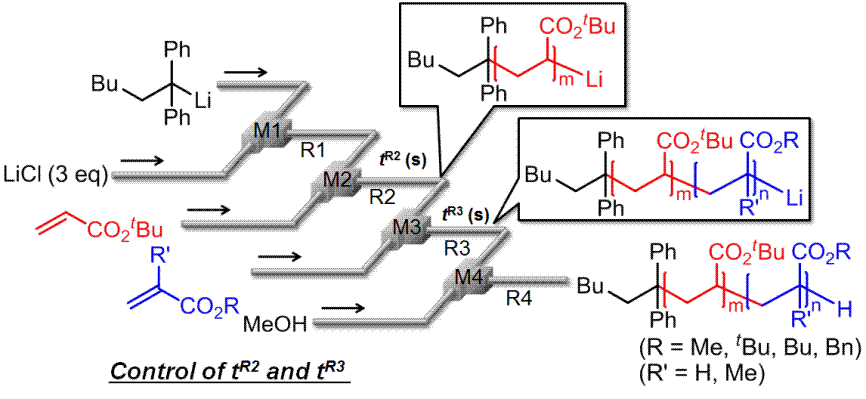
45. Living Anionic Polymerization of tert-Butyl Acrylate in a Flow Microreactor System and Its Applications to the Synthesis of Block Copolymers.
Nagaki, A.; Takahashi, Y.; Akahori, K.; Yoshida, J.
Macromol. React. Eng. 2012, 6, 467–472.
DOI: 10.1002/mren.201200051

44. マイクロリアクターを使った環境調和型有機合成、高分子合成技術
Nagaki, A.; Yoshida, J.
マイクロリアクター技術の最前線(監修:前一廣)
シーエムシー出版、2012年5月17日発刊、ISBN: 978-4-7813-0587-5

43. フローマイクロリアクター合成化学:時間を空間で制御する新しい化学
永木愛一郎、吉田潤一
化学と教育 2012, 60 (5), 190–193.

2011
42. Perfluoroalkylation in Flow Microreactor: Generation of Perfluoroalkyllithiums in the Presence and Absence of Electrophiles.
Nagaki, A.; Tokuoka, S.; Yamada, S.; Tomida, Y.; Oshiro, K.; Amii, H.; Yoshida, J.
Org. Biomol. Chem. 2011, 9, 7559–7563.
DOI: 10.1039/C1OB06350B
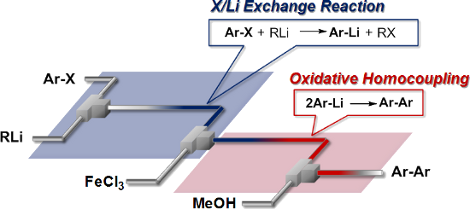
41. Homocoupling of Aryl Halides in Flow: Space Integration of Lithiation and FeCl3 Promoted Homocoupling.
Nagaki, A.; Uesugi, Y.; Tomida, Y.; Yoshida, J.
Beilstein J. Org. Chem. 2011, 7, 1064–1069.
DOI: 10.3762/bjoc.7.122
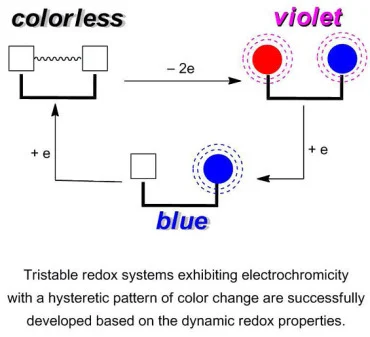
40. Hysteretic Tricolor Electrochromic Systems Based on the Dynamic Redox Properties of Unsymmetrically Substituted Dihydrophenanthrenes and Biphenyl-2,2′-Diyl Dications: Efficient Precursor Synthesis by a Flow Microreactor Method.
Ishigaki, Y.; Suzuki, T.; Nishida, J.; Nagaki, A.; Takabayashi, N.; Kawai, H.; Fujiwara, K.; Yoshida, J.
Materials 2011, 4, 1906–1926.
DOI: 10.3390/ma4111906
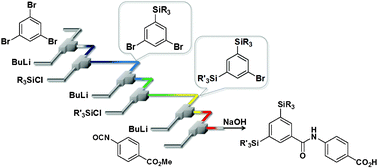
39. Flash Synthesis of TAC-101 and Its Analogues from 1,3,5-Tribromobenzene Using Integrated Flow Microreactor Systems.
Nagaki, A.; Imai, K.; Kim, H.; Yoshida, J.
RSC Adv. 2011, 1, 758–760.
DOI: 10.1039/C1RA00377A
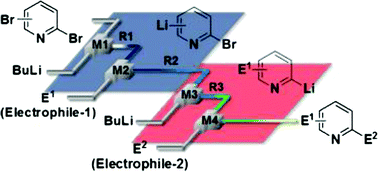
38. Flow Microreactor Synthesis of Disubstituted Pyridines from Dibromopyridines via Br/Li Exchange without Using Cryogenic Conditions.
Nagaki, A.; Yamada, S.; Doi, M.; Tomida, Y.; Takabayashi, N.; Yoshida, J.
Green Chem. 2011, 13, 1110–1113.
DOI: 10.1039/C0GC00852D
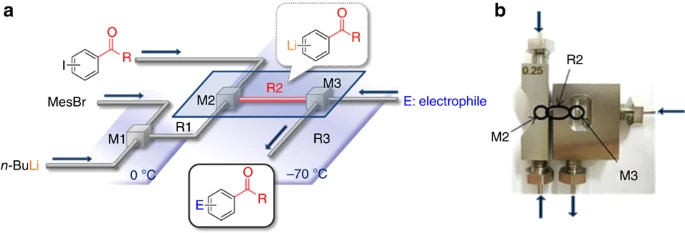
37. A Flow Microreactor Approach to Protecting-group-free Synthesis Using Organolithium Compounds.
Kim, H.; Nagaki, A.; Yoshida, J.
Nat. Commun. 2011, 2, 264.
DOI: 10.1038/ncomms1264
36. Switching Reaction Pathways of Benzo[b]thiophen-3-yllithium and Benzo[b]furan-3-yllithium Based on High-Resolution Residence-Time and Temperature Control in a Flow Microreactor.
Asai, A.; Takata, A.; Ushiogi, Y.; Iinuma, Y.; Nagaki, A.; Yoshida, J.
Chem. Lett. 2011, 40, 393–395.
DOI: 10.1246/cl.2011.393
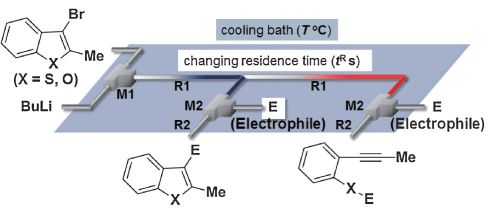
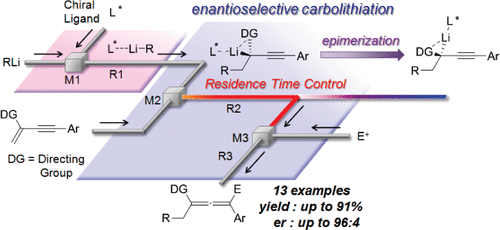
35. Asymmetric Carbolithiation of Conjugated Enynes: A Flow Microreactor Enables the Use of Configurationally Unstable Intermediates before They Epimerize.
Tomida, Y.; Nagaki, A.; Yoshida, J.
J. Am. Chem. Soc. 2011, 133, 3744–3747.
DOI: 10.1021/ja110898s
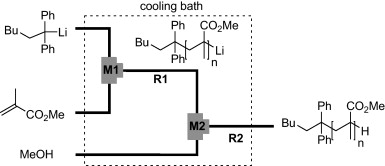
34. Anionic Polymerization of Alkyl Methacrylates using Flow Microreactor Systems.
Nagaki, A.; Miyazaki, A.; Tomida, Y.; Yoshida, J.
Chem. Eng. J. 2011, 167, 548–555.
DOI: 10.1016/j.cej.2010.07.073
33. マイクロリアクターを用いたイオン重合とその精密構造制御ポリマー合成への応用
永木愛一郎
高分子論文集 2011, 68, 521–531.
DOI: 10.1295/koron.68.521

32. マイクロリアクターによる有機リチウム化学の新展開
永木愛一郎、富田裕、吉田潤一
ケミカルエンジニアリング 2011, 56, 54–63.

31. Space Integration of Reactions: An Approach to Increase Capability of Organic Synthesis
Yoshida, J.; Saito, K.; Nokami, T.; Nagaki, A.
Synlett 2011, 9, 1189–1194.
DOI: 10.1055/s-0030-1259946
30. Green and Sustainable Chemical Synthesis Using Flow Microreactors
Yoshida, J.; Kim, H.; Nagaki, A.
ChemSusChem 2011, 4, 331–340.
DOI: 10.1002/cssc.201000271
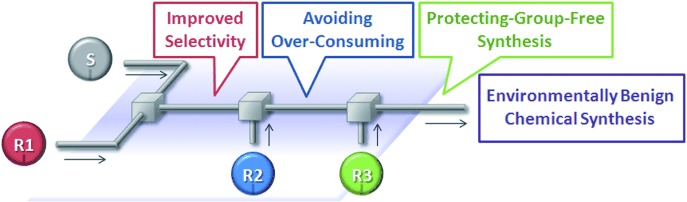

2010
29. Generation and Reactions of Oxiranyllithiums by Use of a Flow Microreactor System.
Nagaki, A.; Takizawa, E.; Yoshida, J.
Chem. Eur. J. 2010, 16, 14149–14158.
DOI: 10.1002/chem.201000815

28. Synthesis of Polystyrenes-Poly(alkyl methacrylates) Block Copolymers via Anionic Polymerization Using an Integrated Flow Microreactor System.
Nagaki, A.; Miyazaki, A.; Yoshida, J.
Macromolecules 2010, 43, 8424–8429.
DOI: 10.1021/ma101663x
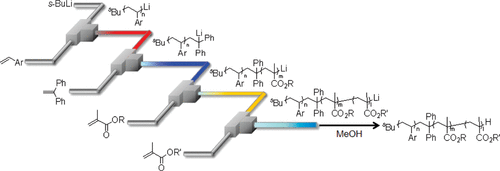
27. A Flow Microreactor System Enables Organolithium Reactions without Protecting Alkoxycarbonyl Groups.
Nagaki, A.; Kim, H.; Moriwaki, Y.; Matsuo, C.; Yoshida, J.
Chem. Eur. J. 2010, 16, 11167–11177.
DOI: 10.1002/chem.201000876
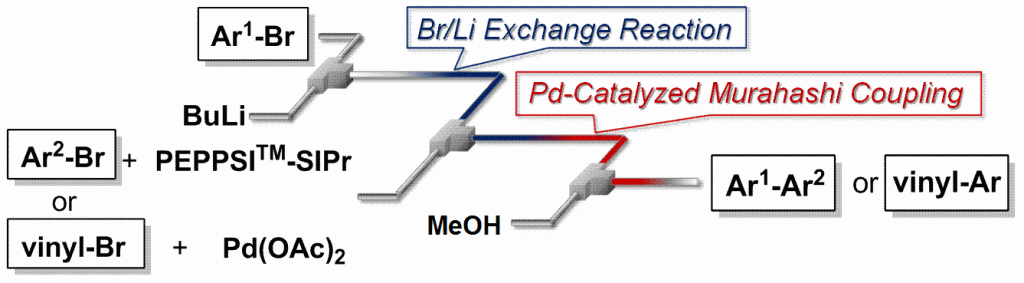
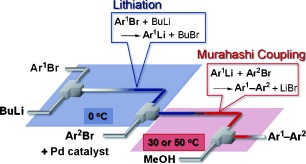
26. Cross-Coupling in a Flow Microreactor: Space Integration of Lithiation and Murahashi Coupling.
Nagaki, A.; Kenmoku, A.; Moriwaki, Y.; Hayashi, A.; Yoshida, J.
Angew. Chem. Int. Ed. 2010, 49, 7543–7547.
DOI: 10.1002/anie.201002763
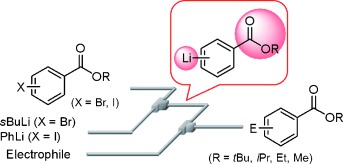
25. Generation and Reaction of Cyano-substituted Aryllithium Compounds Using Microreactors.
Nagaki, A.; Kim, H.; Usutani, H.; Matsuo, C.; Yoshida, J.
Org. Biomol. Chem. 2010, 8, 1212–1217.
DOI: 10.1039/B919325C
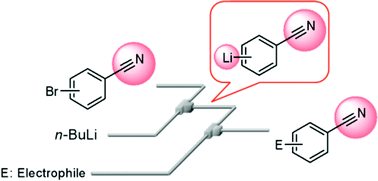
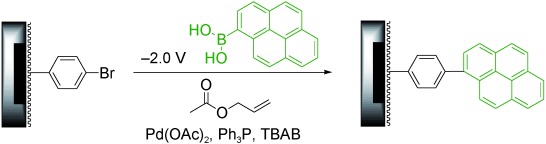
24. Building Addressable Libraries as Platforms for Biological Assays by an Electrochemical Method
Yoshida, J.; Nagaki, A.
Angew. Chem., Int. Ed. 2010, 49, 3720–3722.
DOI: 10.1002/anie.201000046
23. マイクロリアクターを用いた重合反応の制御
永木愛一郎、吉田潤一
高分子 2010, 59, 569–573.

2009
22. Nitro-Substituted Aryl Lithium Compounds in Microreactor Synthesis: Switch between Kinetic and Thermodynamic Control.
Nagaki, A.; Kim, H.; Yoshida, J.
Angew. Chem. Int. Ed. 2009, 48, 8063–8065.
DOI: 10.1002/anie.200904316
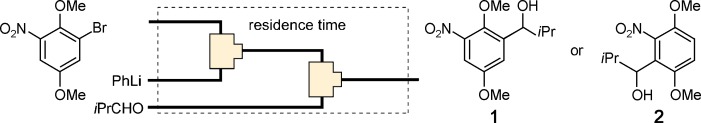
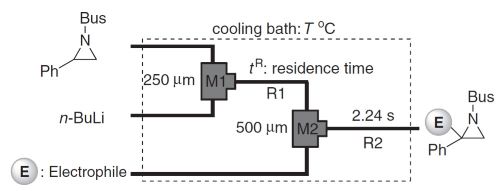
21. Generations and Reactions of N–t-butylsulfonylaziridinyllithiums Using Microreactors.
Nagaki, A.; Takizawa, E.; Yoshida, J.
Chem. Lett. 2009, 38, 1060–1061.
DOI: 10.1246/cl.2009.1060
20. Microflow System Controlled Anionic Polymerization of Alkyl Methacrylates.
Nagaki, A.; Tomida, Y.; Miyazaki, A.; Yoshida, J.
Macromolecules 2009, 42, 4384–4387.
DOI: 10.1021/ma900551a
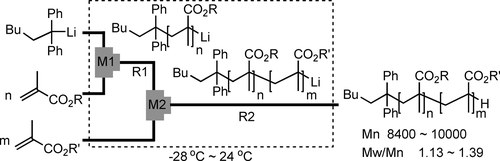
19. Carbolithiation of Conjugated Enynes with Aryllithiums in Microflow System and Applications to Synthesis of Allenylsilanes.
Tomida, Y.; Nagaki, A.; Yoshida, J.
Org. Lett. 2009, 11, 3614–3617.
DOI: 10.1021/ol901352t
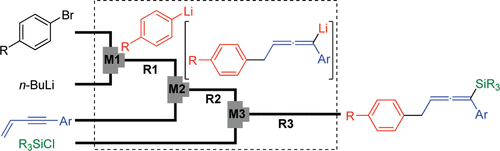
18. Synthesis of Unsymmetrically Substituted Biaryls via Sequential Lithiation of Dibromobiaryls Using Integrated Microflow Systems.
Nagaki, A.; Takabayashi, N.; Tomida, Y.; Yoshida, J.
Beilstein J. Org. Chem. 2009, 5, 16.
DOI: 10.3762/bjoc.5.16
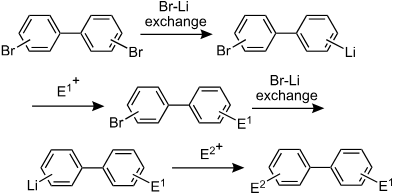
17. Generation and Reactions of alpha-Silyloxiranyllithium in a Microreactor.
Nagaki, A.; Takizawa, E.; Yoshida, J.
Chem. Lett. 2009, 38, 486–487.
DOI: 10.1246/cl.2009.486
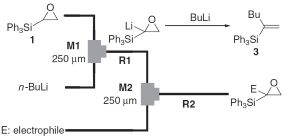
16. Oxiranyl Anion Methodology Using Microflow Systems.
Nagaki, A.; Takizawa, E.; Yoshida, J.
J. Am. Chem. Soc. 2009, 131, 1654–1655 and 3787.
DOI: 10.1021/ja809325a
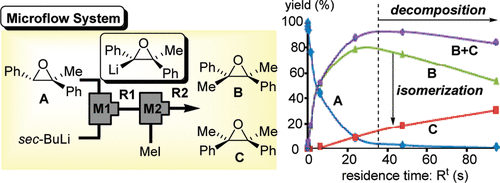
2008
15. Flash Chemistry: Fast Chemical Synthesis by Using Microreactors (concept)
Yoshida, J.; Nagaki, A.; Yamada, T.
Chem. Eur. J. 2008, 14, 7450–7459.
DOI: 10.1002/chem.200800582
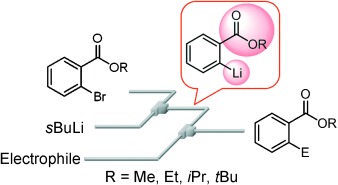
14. Aryllithium Compounds Bearing Alkoxycarbonyl Groups: Generation and Reactions Using a Microflow System.
Nagaki, A.; Kim, H., Yoshida, J.
Angew. Chem. Int. Ed. 2008, 47, 7833–7836.
DOI: 10.1002/anie.200803205
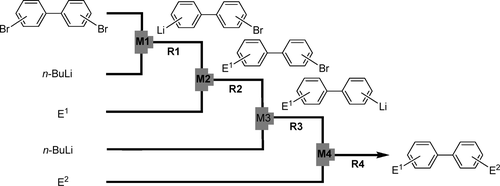
13. Selective Monolithiation of Dibromobiaryls Using Microflow Systems.
Nagaki, A.; Takabayashi, T.; Tomida, Y.; Yoshida, J.
Org. Lett. 2008, 10, 3937–3940.
DOI: 10.1021/ol8015572
12. Microflow System Controlled Anionic Polymerization of Styrenes.
Nagaki, A.; Tomida, Y.; Yoshida, J.
Macromolecules 2008, 17, 6322–6330.
DOI: 10.1021/ma800769n
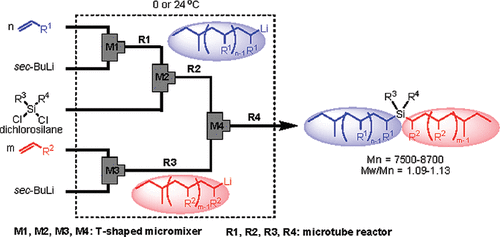
11. Microflow System Controlled Carbocationic Polymerization of Vinyl Ethers.
Nagaki, A.; Iwasaki, T.; Kawamura, K.; Yamada, D.; Suga, S.; Ando, T.; Sawamoto, M.; Yoshida, J.
Chem. Asian J. 2008, 3, 1558–1567.
DOI: 10.1002/asia.200800081
2007
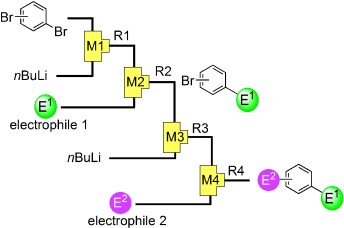
10. Integrated Micro Flow Synthesis Based on Sequential Br-Li Exchange Reactions of p-, m-, and o-Dibromobenzenes.
Nagaki, A.; Tomida, Y.; Usutani, H.; Kim, H.; Takabayashi, N.; Nokami, T.; Okamoto, H.; Yoshida, J.
Chem. Asian J. 2007, 2, 1513–1523.
DOI: 10.1002/asia.200700231
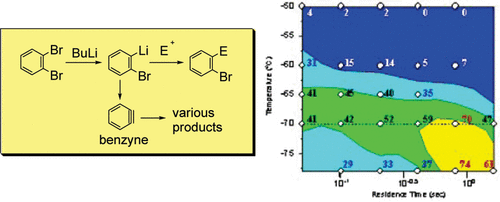
9. Generation and Reactions of o-Bromophenyllithium without Benzyne Formation Using a Microreactor.
Usutani, H.; Tomida, T.; Nagaki, A.; Okamoto, H.; Nokami, T.; Yoshida, J.
J. Am. Chem. Soc. 2007, 129, 3046–3047.
DOI: 10.1021/ja068330s
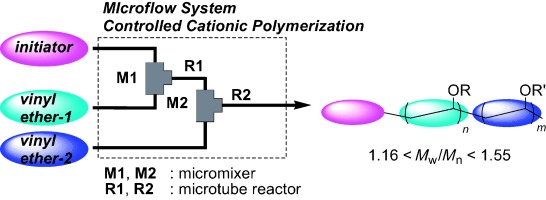
8. Microsystem Controlled Cationic Polymerization of Vinyl Ethers Initiated by CF3SO3H.
Iwasaki, T.; Nagaki, A.; Yoshida, J.
Chem. Commun. 2007, 1263–1265.
DOI: 10.1039/b615159k
before 2006
7. Control of Extremely Fast Competitive Consecutive Reactions Using Micromixing. Selective Friedel-Crafts Aminoalkylation.
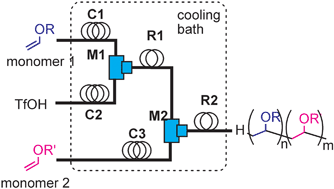
Nagaki, A.; Togai, M.; Suga, S.; Aoki, N.; Mae, K.; Yoshida, J.
J. Am. Chem. Soc. 2005, 127, 11666–11675.
DOI: 10.1021/ja0527424
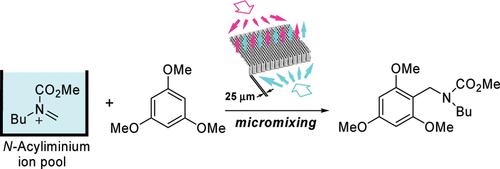
6. Cycloaddition of “N-Acyliminium Ion Pools” with Carbon-Carbon Multiple Bonds.
Suga, S.; Tsutsui, Y.; Nagaki, A.; Yoshida, J.
Bull. Chem. Soc. Jpn. 2005, 78, 1206–1271.
DOI: 10.1246/bcsj.78.1206
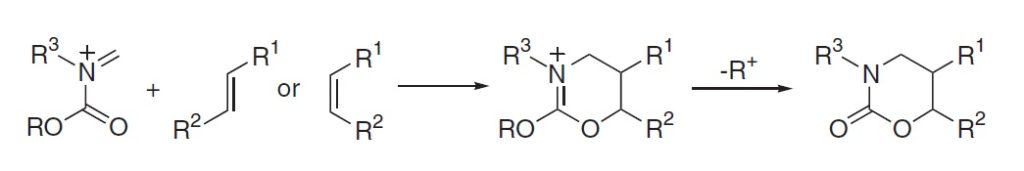
5. マイクロリアクターを用いた選択的有機反応
吉田潤一、菅誠治、永木愛一郎
有機合成化学協会誌 2005, 63, 511–522.
DOI: 10.5059/yukigoseikyokaishi.63.511

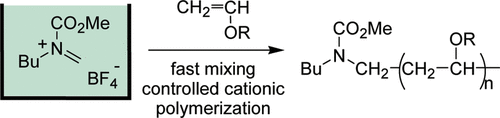
4. “Cation Pool” Initiated Controlled/Living Polymerization Using Microsystems.
Nagaki, A.; Kawamura, K.; Suga, S.; Ando, T.; Sawamoto, M.; Yoshida, J.
J. Am. Chem. Soc. 2004, 126, 14702–14703.
DOI: 10.1021/ja044879k
3. Three-Component Coupling Based on the “Cation Pool” Method.
Suga, S.; Nishida, T.; Yamada, D.; Nagaki, A.; Yoshida, J.
J. Am. Chem. Soc. 2004, 126, 14338–14339.
DOI: 10.1021/ja0455704
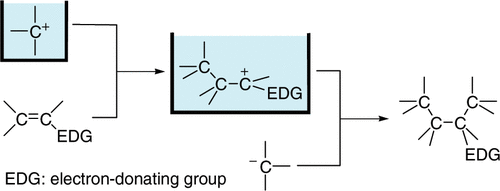
2. “N-Acyliminium Ion Pool” as Hetero Diene in [4+2] Cycloaddition Reaction.
Suga, S.; Nagaki, A.; Tsutsui, Y.; Yoshida, J.
Org. Lett. 2003, 5, 945–947.
DOI: 10.1021/ol0341243
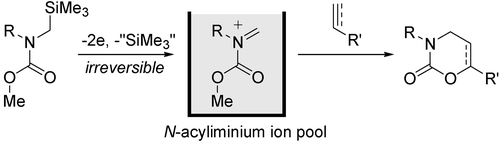
1. Highly Selective Friedel-Crafts Monoalkylation Using Micromixing.
Suga, S.; Nagaki, A.; Yoshida, J.
Chem. Commun. 2003, 354–355.
DOI: 10.1039/b211433j